Prognostic Role of Pre-Treatment Body Composition Parameters in Patients Undergoing First-Line Immunotherapy for Metastatic Renal Cell Carcinoma
- PMID: 39220816
- PMCID: PMC11366243
- DOI: 10.2147/CMAR.S476150
Prognostic Role of Pre-Treatment Body Composition Parameters in Patients Undergoing First-Line Immunotherapy for Metastatic Renal Cell Carcinoma
Abstract
Purpose: We investigated the relationship between body mass index (BMI), radiological body composition, and survival outcomes in patients with metastatic renal cell carcinoma (mRCC) underwent first-line immune checkpoint inhibitor (ICI)-based therapy.
Methods: Analyzing data from 102 patients treated between November 2019 and March 2023, pre-treatment computed tomography (CT) scans assessed fat and muscle areas. BMI and body composition indices were examined, including skeletal muscle index, subcutaneous fat index (SFI), visceral fat index, and total fat index. Kaplan-Meier curves and Log rank tests compared progression-free survival (PFS) and overall survival (OS), while multivariable Cox proportional regression analysis was performed to identify the variables significantly associated with survival outcomes.
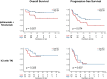
Results: 54 patients (52.9%) experienced disease progression, and 26 (25.5%) died during a median follow-up of 17.4 months. High SFI was significantly associated with improved OS (p = 0.018) but not PFS (p = 0.090). Multivariable analysis confirmed the positive impact of high SFI on OS (adjusted HR: 0.37, p = 0.029) and suggested a trend towards improved PFS (adjusted HR: 0.61, p = 0.088). Notably, in the ipilimumab + nivolumab subgroup, high SFI significantly correlated with both PFS and OS (p = 0.047 and p = 0.012, respectively).
Conclusion: High SFI predicts favorable OS in patients with mRCC receiving first-line ICI-based therapy, especially patients treated with ipilimumab + nivolumab displayed a significant association between high SFI and favorable PFS and OS.
Keywords: body composition; immunotherapy; prognosis; renal cell carcinoma.
© 2024 Lee et al.
Conflict of interest statement
The authors declare no competing interests in this work.
Figures


Similar articles
-
Body Composition Variables as Radiographic Biomarkers of Clinical Outcomes in Metastatic Renal Cell Carcinoma Patients Receiving Immune Checkpoint Inhibitors.Front Oncol. 2021 Jul 9;11:707050. doi: 10.3389/fonc.2021.707050. eCollection 2021. Front Oncol. 2021. PMID: 34307176 Free PMC article.
-
Relationship Between Pretreatment Body Composition and Clinical Outcomes in Patients With Metastatic Renal Cell Carcinoma Receiving First-Line Ipilimumab Plus Nivolumab.Clin Genitourin Cancer. 2023 Dec;21(6):e429-e437.e2. doi: 10.1016/j.clgc.2023.05.006. Epub 2023 May 18. Clin Genitourin Cancer. 2023. PMID: 37271698
-
Association between Immune Checkpoint Inhibitor Treatment Outcomes and Body Composition Factors in Metastatic Renal Cell Carcinoma Patients.Cancers (Basel). 2023 Nov 26;15(23):5591. doi: 10.3390/cancers15235591. Cancers (Basel). 2023. PMID: 38067295 Free PMC article.
-
Prognostic value of body composition on survival outcomes in melanoma patients receiving immunotherapy.Front Immunol. 2023 Nov 22;14:1261202. doi: 10.3389/fimmu.2023.1261202. eCollection 2023. Front Immunol. 2023. PMID: 38077332 Free PMC article.
-
Targeted therapy for metastatic renal cell carcinoma.Cochrane Database Syst Rev. 2020 Oct 14;10(10):CD012796. doi: 10.1002/14651858.CD012796.pub2. Cochrane Database Syst Rev. 2020. PMID: 33058158 Free PMC article.
Cited by
-
Impact of body composition on pathological response to neoadjuvant immunotherapy in dMMR/MSI-H colorectal cancer.Front Immunol. 2025 May 30;16:1589869. doi: 10.3389/fimmu.2025.1589869. eCollection 2025. Front Immunol. 2025. PMID: 40519904 Free PMC article.
References
-
- Santoni M, Buti S, Myint ZW, et al. Real-world outcome of patients with advanced renal cell carcinoma and intermediate- or poor-risk international metastatic renal cell carcinoma database consortium criteria treated by immune-oncology combinations: differential effectiveness by risk group? Eur Urol Oncol. 2023;7:102–111. doi:10.1016/j.euo.2023.07.003 - DOI - PubMed
LinkOut - more resources
Full Text Sources

