Probing the Distribution and Mobility of Aminopolymers after Multiple Sorption-Regeneration Cycles: Neutron Scattering Studies
- PMID: 39220859
- PMCID: PMC11363015
- DOI: 10.1021/acs.iecr.4c01595
Probing the Distribution and Mobility of Aminopolymers after Multiple Sorption-Regeneration Cycles: Neutron Scattering Studies
Abstract
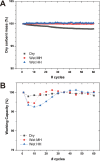
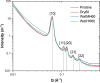
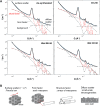
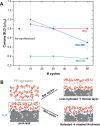
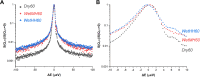
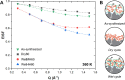
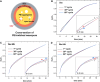
Solid-supported amines are effective CO2 adsorbents capable of capturing CO2 from flue gas streams (10-15 vol % CO2) and from ultradilute streams, such as ambient air (∼400 ppm CO2). Amine sorbents have demonstrated promising performance (e.g., high CO2 uptake and uptake rates) with stable characteristics under repeated, idealized thermal swing conditions, enabling multicycle application. Literature studies suggest that solid-supported amines such as PEI/SBA-15 generally exhibit slowly reducing CO2 uptake rates or capacities over repeated thermal swing capture-regeneration cycles under simulated DAC conditions. While there are experimental reports describing changes in supported amine mass, degradation of amine sites, and changes in support structures over cycling, there is limited knowledge about the structure and mobility of the amine domains in the support pores over extended use. Furthermore, little is known about the effects of H2O on cyclic applications of PEI/SBA-15 despite the inevitable presence of H2O in ambient air. Here, we present a series of neutron scattering studies exploring the distribution and mobility of PEI in mesoporous silica SBA-15 as a function of thermal cycling and cyclic conditions. Small-angle neutron scattering (SANS) and quasielastic neutron scattering (QENS) are used to study the amine and H2O distributions and amine mobility, respectively. Applying repeated thermal swings under dry conditions leads to the thorough removal of water from the sorbent, causing thinner and more rigid wall-coating PEI layers that eventually lead to slower CO2 uptake rates. On the other hand, wet cyclic conditions led to the sorption of atmospheric water at the wall-PEI interfaces. When PEI remains hydrated, the amine distribution (i.e., wall-coating PEI layer thickness) is retained over cycling, while lubrication effects of water yield improved PEI mobility, in turn leading to faster CO2 uptake rates.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): C.W.J. has a financial interest in several companies that seek to commercialize CO2 capture from air. C.W.J. has a conflict-of-interest management plan in place at Georgia Tech.
Figures
Similar articles
-
Distribution and Mobility of Amines Confined in Porous Silica Supports Assessed via Neutron Scattering, NMR, and MD Simulations: Impacts on CO2 Sorption Kinetics and Capacities.Acc Chem Res. 2023 Oct 3;56(19):2620-2630. doi: 10.1021/acs.accounts.3c00363. Epub 2023 Sep 18. Acc Chem Res. 2023. PMID: 37722889 Free PMC article.
-
Underlying Roles of Polyol Additives in Promoting CO2 Capture in PEI/Silica Adsorbents.ChemSusChem. 2024 Nov 25;17(22):e202400967. doi: 10.1002/cssc.202400967. Epub 2024 Aug 2. ChemSusChem. 2024. PMID: 38830830 Free PMC article.
-
Direct Air Capture of CO2 Using Poly(ethyleneimine)-Functionalized Expanded Poly(tetrafluoroethylene)/Silica Composite Structured Sorbents.ACS Appl Mater Interfaces. 2022 Sep 14;14(36):40992-41002. doi: 10.1021/acsami.2c11143. Epub 2022 Sep 1. ACS Appl Mater Interfaces. 2022. PMID: 36047596
-
Direct Capture of CO2 from Ambient Air.Chem Rev. 2016 Oct 12;116(19):11840-11876. doi: 10.1021/acs.chemrev.6b00173. Epub 2016 Aug 25. Chem Rev. 2016. PMID: 27560307 Review.
-
Amine-Functionalized Cellulose as Promising Materials for Direct CO2 Capture: A Review.ACS Appl Mater Interfaces. 2025 Mar 19;17(11):16380-16395. doi: 10.1021/acsami.4c20801. Epub 2025 Mar 4. ACS Appl Mater Interfaces. 2025. PMID: 40038886 Review.
References
-
- Hammache S.; Hoffman J. S.; Gray M. L.; Fauth D. J.; Howard B. H.; Pennline H. W. Comprehensive Study of the Impact of Steam on Polyethyleneimine on Silica for CO2 Capture. Energy Fuels 2013, 27 (11), 6899–6905. 10.1021/ef401562w. - DOI
-
- Reynolds A. J.; Verheyen T. V.; Adeloju S. B.; Meuleman E.; Feron P. Towards Commercial Scale Postcombustion Capture of CO2 with Monoethanolamine Solvent: Key Considerations for Solvent Management and Environmental Impacts. Environ. Sci. Technol. 2012, 46 (7), 3643–3654. 10.1021/es204051s. - DOI - PubMed
-
- Darunte L. A.; Oetomo A. D.; Walton K. S.; Sholl D. S.; Jones C. W. Direct Air Capture of CO2 Using Amine Functionalized MIL-101 (Cr). ACS Sustain. Chem. Eng. 2016, 4 (10), 5761–5768. 10.1021/acssuschemeng.6b01692. - DOI
-
- Harlick P. J.; Sayari A. Applications of Pore-Expanded Mesoporous Silicas. 3. Triamine Silane Grafting for Enhanced CO2 Adsorption. Ind. Eng. Chem. Res. 2006, 45 (9), 3248–3255. 10.1021/ie051286p. - DOI
LinkOut - more resources
Full Text Sources
