Ailanthone ameliorates pulmonary fibrosis by suppressing JUN-dependent MEOX1 activation
- PMID: 39220862
- PMCID: PMC11365432
- DOI: 10.1016/j.apsb.2024.04.013
Ailanthone ameliorates pulmonary fibrosis by suppressing JUN-dependent MEOX1 activation
Abstract
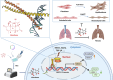
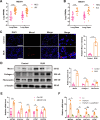
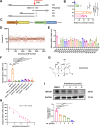
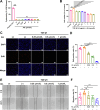
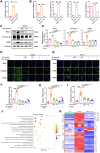
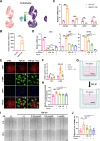
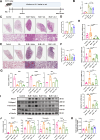
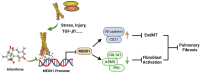
Pulmonary fibrosis poses a significant health threat with very limited therapeutic options available. In this study, we reported the enhanced expression of mesenchymal homobox 1 (MEOX1) in pulmonary fibrosis patients, especially in their fibroblasts and endothelial cells, and confirmed MEOX1 as a central orchestrator in the activation of profibrotic genes. By high-throughput screening, we identified Ailanthone (AIL) from a natural compound library as the first small molecule capable of directly targeting and suppressing MEOX1. AIL demonstrated the ability to inhibit both the activation of fibroblasts and endothelial-to-mesenchymal transition of endothelial cells when challenged by transforming growth factor-β1 (TGF-β1). In an animal model of bleomycin-induced pulmonary fibrosis, AIL effectively mitigated the fibrotic process and restored respiratory functions. Mechanistically, AIL acted as a suppressor of MEOX1 by disrupting the interaction between the transcription factor JUN and the promoter of MEOX1, thereby inhibiting MEOX1 expression and activity. In summary, our findings pinpointed MEOX1 as a cell-specific and clinically translatable target in fibrosis. Moreover, we demonstrated the potent anti-fibrotic effect of AIL in pulmonary fibrosis, specifically through the suppression of JUN-dependent MEOX1 activation.
Keywords: Ailanthone; High-throughput screening; JUN; MEOX1; Natural product; Pulmonary fibrosis; TGF-β1.
© 2024 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest exist.
Figures

References
-
- Tzilas V., Tzouvelekis A., Ryu J.H., Bouros D. 2022 update on clinical practice guidelines for idiopathic pulmonary fibrosis and progressive pulmonary fibrosis. Lancet Respir Med. 2022;10:729–731. - PubMed
-
- King T.J., Bradford W.Z., Castro-Bernardini S., Fagan E.A., Glaspole I., Glassberg M.K., et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–2092. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

