Evaluation of analytical greenness metric for an eco-friendly method developed through the integration of green chemistry and quality-by-design for the simultaneous determination of Nebivolol hydrochloride, Telmisartan, Valsartan, and Amlodipine besylate
- PMID: 39220975
- PMCID: PMC11365310
- DOI: 10.1016/j.heliyon.2024.e35376
Evaluation of analytical greenness metric for an eco-friendly method developed through the integration of green chemistry and quality-by-design for the simultaneous determination of Nebivolol hydrochloride, Telmisartan, Valsartan, and Amlodipine besylate
Abstract
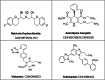
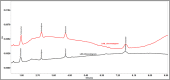
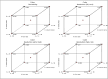
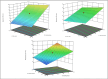
In recent years, the field of analytical chemistry has witnessed a notable shift towards the adoption of greener chromatographic methods, aiming to minimize the environmental impact. An effective strategy involves substituting conventional harmful organic solvents with environmentally friendly alternatives, reducing the use of hazardous chemicals that contribute to environmental concerns. However, separating drug substances without the use of buffers and organic solvents presence is a big challenge. To overcome this challenge, a combination of quality-by-design (QbD) and green analytical chemistry (GAC) was employed in this study for method development. A high-performance liquid chromatography (HPLC) method was successfully developed and validated for the simultaneous determination of Nebivolol hydrochloride, Telmisartan, Valsartan, and Amlodipine besylate. The method utilized a mobile phase composed of a mixture of 0.1 % formic acid in water (pH: 2.5) and ethanol. A regular octadecyl silica (ODS) column was employed, and UV detection at 220 nm was utilized. The method exhibited linearity within the concentration range of 25-75 μg/mL for Telmisartan and 150-450 μg/mL for Nebivolol Hydrochloride, Valsartan, and Amlodipine besylate and the correlation coefficient was greater than 0.999 for all the analytes. Limits of detection (LOD) and quantification (LOQ) were determined as 0.01 and 0.04 μg/mL for Telmisartan, 0.06 and 0.20 μg/mL for Nebivolol Hydrochloride, 0.08 and 0.25 μg/mL for Amlodipine besylate, and 0.14 and 0.46 μg/mL for Valsartan, respectively. The developed method underwent thorough validation, encompassing various parameters such as linearity, accuracy, precision, LOD, LOQ, robustness, and ruggedness. The mean recovery values were observed to range between 98.86 % and 99.89 %. The accuracy demonstrated was consistently above 98.98 % for both intra-day and inter-day precisions were with the relative standard deviations less than 2 %. To establish its robustness, a quality-by-design-based experimental design (DoE) approach was implemented. Additionally, the method's environmental friendliness was evaluated using the Analytical Greenness metric (AGREE) an analytical eco scale, both confirming its alignment with sustainable practices and reduced ecological impact. The sustainability of the solvent used in the current study was evaluated by Green Solvents Selecting Tool (GSST) Further, the developed method greenness was evaluated with the green analytical tools such as Analytical method greenness score (AMGS) and using the recently released White Analytical Chemistry (WAC) using RGB assessment tool. By employing this greener approach to chromatography method, this study contributes to the ongoing efforts in analytical chemistry to promote sustainable practices and minimize the environmental footprint of analytical methods.
Keywords: Amlodipine; Design of experiments; Green analytical chemistry; Nebivolol hydrochloride; Quality-by-Design; Telmisartan; Validation; Valsartan.
© 2024 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Dr. Pradeep Kumar Brahman reports was provided by KL Deemed to be University. Dr. Pradeep Kumar Brahman reports a relationship with KL Deemed to be University Department of Chemistry that includes: employment. Dr. Pradeep Kumar Brahman has patent no Patent pending to NOT APPLICABLE. No other relationship or activity with publisher If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








References
-
- Salim Mohamed M., Radwan Aya saad, Hadad Ghada M., Belal Fathalla, Elkhoudary Mahmoud M. Green fluorometric strategy for simultaneous determination of the antihypertensive drug telmisartan (A tentative therapeutic for COVID-19) with Nebivolol in human plasma. Sci. Rep. 2023;13:3576. doi: 10.1038/s41598-023-30400-w. - DOI - PMC - PubMed
-
- Rajoriya Vaibhav, Kashaw Varsha. RP-HPLC method for the simultaneous estimation of nebivolol hydrochloride and valsartan. Analytical Chemistry Letters. 2017;7(4):520–530. doi: 10.1080/22297928.2017.1362994. - DOI
-
- Nalwade S., Ranga Reddy V., Durga Rao D., Koteswara Rao I. Rapid simultaneous determination of telmisartan, amlodipine besylate and hydrochlorothiazide in a combined poly pill dosage form by stability-indicating ultra performance liquid chromatography. Sci. Pharm. 2011 Jan-Mar;79(1):69–84. doi: 10.3797/scipharm.1006-10. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

