Ferroptosis in liver diseases: Fundamental mechanism and clinical implications
- PMID: 39221065
- PMCID: PMC11362879
- DOI: 10.3748/wjg.v30.i32.3730
Ferroptosis in liver diseases: Fundamental mechanism and clinical implications
Abstract
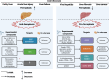
This editorial discusses a recently published paper in the World Journal of Gastroenterology. Our research focuses on p53's regulatory mechanism for controlling ferroptosis, as well as the intricate connection between ferroptosis and liver diseases. Ferroptosis is a specific form of programmed cell death that is de-pendent on iron and displays unique features in terms of morphology, biology, and genetics, distinguishing it from other forms of cell death. Ferroptosis can affect the liver, which is a crucial organ responsible for iron storage and meta-bolism. Mounting evidence indicates a robust correlation between ferroptosis and the advancement of liver disorders. P53 has a dual effect on ferroptosis through various distinct signaling pathways. However, additional investigations are required to clarify the regulatory function of p53 metabolic targets in this complex association with ferroptosis. In the future, researchers should clarify the mechanisms by which ferroptosis and other forms of programmed cell death contribute to the progression of liver diseases. Identifying and controlling important regulatory factors associated with ferroptosis present a promising therapeutic strategy for liver disorders.
Keywords: Ferroptosis; Liver disease; P53; Programmed cell death; Therapeutic target.
©The Author(s) 2024. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: There is no conflict of interest associated with any of authors who contributed their efforts in this manuscript.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

