Dual roles of photosynthetic hydrogel with sustained oxygen generation in promoting cell survival and eradicating anaerobic infection
- PMID: 39221211
- PMCID: PMC11364899
- DOI: 10.1016/j.mtbio.2024.101197
Dual roles of photosynthetic hydrogel with sustained oxygen generation in promoting cell survival and eradicating anaerobic infection
Abstract
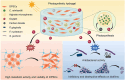
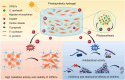
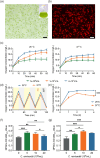
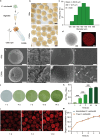
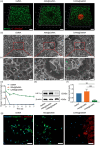
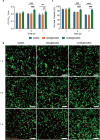
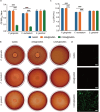
Tissue engineering offers a promising alternative for oral and maxillofacial tissue defect rehabilitation; however, cells within a sizeable engineered tissue construct after transplantation inevitably face prolonged and severe hypoxic conditions, which may compromise the survivability of the transplanted cells and arouse the concern of anaerobic infection. Microalgae, which can convert carbon dioxide and water into oxygen and glucose through photosynthesis, have been studied as a source of oxygen supply for several biomedical applications, but their promise in orofacial tissue regeneration remains unexplored. Here, we demonstrated that through photosynthetic oxygenation, Chlamydomonas reinhardtii (C. reinhardtii) supported dental pulp stem cell (DPSC) energy production and survival under hypoxia. We developed a multifunctional photosynthetic hydrogel by embedding DPSCs and C. reinhardtii encapsulated alginate microspheres (CAMs) within gelatin methacryloyl hydrogel (GelMA) (CAMs@GelMA). This CAMs@GelMA hydrogel can generate a sustainable and sufficient oxygen supply, reverse intracellular hypoxic status, and enhance the metabolic activity and viability of DPSCs. Furthermore, the CAMs@GelMA hydrogel exhibited selective antibacterial activity against oral anaerobes and remarkable antibiofilm effects on multispecies biofilms by disrupting the hypoxic microenvironment and increasing reactive oxygen species generation. Our work presents an innovative photosynthetic strategy for oral tissue engineering and opens new avenues for addressing other hypoxia-related challenges.
Keywords: Anaerobic bacteria; Antibacterial activity; Chlamydomonas reinhardtii; Oxygen; Photosynthesis; Tissue engineering.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
In Situ Oxygen Generation Enhances the SCAP Survival in Hydrogel Constructs.J Dent Res. 2021 Sep;100(10):1127-1135. doi: 10.1177/00220345211027155. Epub 2021 Jul 30. J Dent Res. 2021. PMID: 34328028
-
Regulation of the fate of dental-derived mesenchymal stem cells using engineered alginate-GelMA hydrogels.J Biomed Mater Res A. 2017 Nov;105(11):2957-2967. doi: 10.1002/jbm.a.36148. Epub 2017 Jul 14. J Biomed Mater Res A. 2017. PMID: 28639378 Free PMC article.
-
[Islet biomimetic microenvironment constructed by chitosan oligosaccharide protects islets from hypoxia-induced damage by reducing intracellular reactive oxygen species].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2022 May 15;36(5):633-642. doi: 10.7507/1002-1892.202201063. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2022. PMID: 35570640 Free PMC article. Chinese.
-
Photosynthetic H2 metabolism in Chlamydomonas reinhardtii (unicellular green algae).Planta. 2007 Oct;226(5):1075-86. doi: 10.1007/s00425-007-0609-9. Epub 2007 Aug 25. Planta. 2007. PMID: 17721788 Review.
-
Recent Advances on Bioprinted Gelatin Methacrylate-Based Hydrogels for Tissue Repair.Tissue Eng Part A. 2021 Jun;27(11-12):679-702. doi: 10.1089/ten.TEA.2020.0350. Epub 2021 Mar 9. Tissue Eng Part A. 2021. PMID: 33499750 Review.
References
-
- Zhang Q., Yang T., Zhang R., Liang X., Wang G., Tian Y., Xie L., Tian W. Platelet lysate functionalized gelatin methacrylate microspheres for improving angiogenesis in endodontic regeneration. Acta Biomater. 2021;136:441–455. - PubMed
-
- Cheng G., Guo S., Li M., Xiao S., Jiang B., Ding Y. Hydroxyapatite-coated small intestinal submucosa membranes enhanced periodontal tissue regeneration through immunomodulation and osteogenesis via BMP-2/Smad signaling pathway. Adv. Healthc. Mater. 2023;13 - PubMed
-
- Luo J., Chen H., Wang G., Lyu J., Liu Y., Lin S., Zhou M., Jiang X. CGRP-loaded porous microspheres protect BMSCs for alveolar bone regeneration in the periodontitis microenvironment. Adv. Healthc. Mater. 2023;12 - PubMed
-
- Wang H., Xu Y., Wang P., Ma J., Wang P., Han X., Fan Y., Bai D., Sun Y., Zhang X. Cell-mediated injectable blend hydrogel-BCP ceramic scaffold for in situ condylar osteochondral repair. Acta Biomater. 2021;123:364–378. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

