E-cigarette exposure disrupts antitumor immunity and promotes metastasis
- PMID: 39221247
- PMCID: PMC11365074
- DOI: 10.3389/fimmu.2024.1444020
E-cigarette exposure disrupts antitumor immunity and promotes metastasis
Abstract
Electronic cigarettes (e-cigarettes) are thought to pose low risk of cancer because the components of e-cigarette liquid are not carcinogens. We analyzed the effects of the two major components, PG/VG and nicotine, on tumor development in preclinical models. We found that PG/VG promoted tumor cell migration in migration assays and contributed to more aggressive, metastatic, and immunosuppressive tumors in vivo, aggravated by the presence of nicotine. Whole body exposure of mice to PG/VG and nicotine rendered animals more susceptible to developing tumors with high frequencies of infiltrating proinflammatory macrophages expressing IL-6 and TNFα. Moreover, tumor-infiltrating and circulating T cells in e-cigarette exposed mice showed increased levels of immune checkpoints including CTLA4 and PD-1. Treatment with anti-CTLA4 antibody was able to abrogate metastasis with no detrimental effects on its ability to induce tumor regression in exposed mice. These findings suggest that the major components used in e-cigarette fluid can impact tumor development through induced immunosuppression.
Keywords: electronic cigarettes; immune checkpoint blockade; immunosuppression; metastasis; whole body exposure.
Copyright © 2024 Arias-Badia, Pai, Chen, Chang, Lwin, Srinath, Gotts, Glantz and Fong.
Conflict of interest statement
LF received research funding from Abbvie, Bavarian Nordic, Bristol-Myers Squibb, Dendreon, Janssen, Merck, Nektar, Roche/Genentech and Parker Institute; served as a consultant to Abbvie, Actym, Amgen, Astra Zeneca, Bioatla, Bolt, Bristol Myer Squibb, Crescendo, Daiichi Sankyo, Immunogenesis, Innovent, Merck, NGMBio, Nutcracker, RAPT, Senti, Sutro, and Roche/Genentech; and has ownership interests in Actym, Bioatla, Bolt, Immunogenesis, Nutcracker, RAPT, and Senti, unrelated to the work here. SAG served as a consultant to the World Health Organization for work unrelated to this project. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

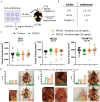
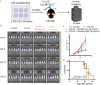



References
-
- Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems. Board on Population Health and Public Health Practice. Health and Medicine Division. National Academies of Sciences. Engineering, and Medicine . Public health consequences of E-cigarettes. Stratton K, Kwan LY, Eaton DL, editors. Washington, D.C: National Academies Press; (2018). Available at: https://www.nap.edu/catalog/24952. - PubMed
-
- Broderick JJ, Brumburgh F, Krum JK, Laughlin JL. Recent progress in the consideration of flavc/ring ingredients under the food additives amendment. Food Technol. (1965).
-
- McNeill A. E-cigarettes: an evidence update. London, UK: Public Health England; (2015).

