Preservation of functionality, immunophenotype, and recovery of HIV RNA from PBMCs cryopreserved for more than 20 years
- PMID: 39221258
- PMCID: PMC11361978
- DOI: 10.3389/fimmu.2024.1382711
Preservation of functionality, immunophenotype, and recovery of HIV RNA from PBMCs cryopreserved for more than 20 years
Abstract
Background: Many research laboratories have long-term repositories of cryopreserved peripheral blood mononuclear cells (PBMC), which are costly to maintain but are of uncertain utility for immunological studies after decades in storage. This study investigated preservation of cell surface phenotypes and in-vitro functional capacity of PBMC from viraemic HIV+ patients and healthy seronegative control subjects, after more than 20 years of cryopreservation.
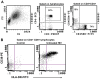
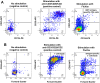
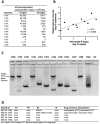
Methods: PBMC were assessed by 18-colour flow cytometry for major lymphocyte subsets within T, B, NK, and dendritic cells and monocytes. Markers of T-cell differentiation and activation were compared with original immunophenotyping performed in 1995/1996 on fresh blood at the time of collection. Functionality of PBMC was assessed by culture with influenza antigen or polyclonal T-cell activation, to measure upregulation of activation-induced CD25 and CD134 (OX40) on CD4 T cells and cytokine production at day 2, and proliferative CD25+ CD4 blasts at day 7. RNA was extracted from cultures containing proliferating CD4+ blast cells, and intracellular HIV RNA was measured using short amplicons for both the Double R and pol region pi code assays, whereas long 4-kbp amplicons were sequenced.
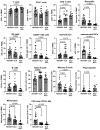
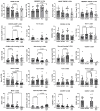
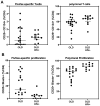
Results: All major lymphocyte and T-cell subpopulations were conserved after long-term cryostorage, except for decreased proportions of activated CD38+HLA-DR+ CD4 and CD8 T cells in PBMC from HIV+ patients. Otherwise, differences in T-cell subpopulations between recent and long-term cryopreserved PBMC primarily reflected donor age-associated or HIV infection-associated effects on phenotypes. Proportions of naïve, memory, and effector subsets of T cells from thawed PBMC correlated with results from the original flow cytometric analysis of respective fresh blood samples. Antigen-specific and polyclonal T-cell responses were readily detected in cryopreserved PBMC from HIV+ patients and healthy control donors. Intracellular HIV RNA quantitation by pi code assay correlated with original plasma viral RNA load results. Full-length intracellular and supernatant-derived amplicons were generated from 5/12 donors, and sequences were ≥80% wild-type, consistent with replication competence.
Conclusions: This unique study provides strong rationale and validity for using well-maintained biorepositories to support immunovirological research even decades after collection.
Keywords: HIV reactivation; T cell function; T cell subsets; biobank; cryopreservation; immunophenotyping; memory T cells; viability.
Copyright © 2024 Dyer, Suzuki, Levert, Starr, Lloyd and Zaunders.
Conflict of interest statement
KS is the original inventor under WO2018/045425 PTC/ AU2017/050974 patent, titled “Methods of detecting Lentivirus” of HIV-1 detection targeting “R” region. KS and AL are the original inventors under Australian Provisional Patent Application 2023903113, titled “Molecular assay for detecting lentivirus” of HIV-1 detection targeting “Pol-A1 region”. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships thatcould be construed as a potential conflict of interest.
Figures




References
-
- Olson WC, Smolkin ME, Farris EM, Fink RJ, Czarkowski AR, Fink JH, et al. Shipping blood to a central laboratory in multicenter clinical trials: effect of ambient temperature on specimen temperature, and effects of temperature on mononuclear cell yield, viability and immunologic function. J Transl Med. (2011) 9:26. doi: 10.1186/1479-5876-9-26 - DOI - PMC - PubMed
-
- Posevitz-Fejfár A, Posevitz V, Gross CC, Bhatia U, Kurth F, Schütte V, et al. Effects of blood transportation on human peripheral mononuclear cell yield, phenotype and function: implications for immune cell biobanking. PloS One. (2014) 9:e115920. doi: 10.1371/journal.pone.0115920 - DOI - PMC - PubMed
-
- Sarzotti-Kelsoe M, Needham LK, Rountree W, Bainbridge J, Gray CM, Fiscus SA, et al. The Center for HIV/AIDS Vaccine Immunology (CHAVI) multi-site quality assurance program for cryopreserved human peripheral blood mononuclear cells. J Immunol Methods. (2014) 409:21–30. doi: 10.1016/j.jim.2014.05.013 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

