The role of DNA topoisomerase 1α (AtTOP1α) in regulating arabidopsis meiotic recombination and chromosome segregation
- PMID: 39221285
- PMCID: PMC11365474
- DOI: 10.7717/peerj.17864
The role of DNA topoisomerase 1α (AtTOP1α) in regulating arabidopsis meiotic recombination and chromosome segregation
Abstract
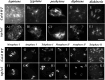
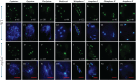
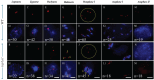
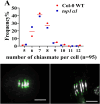
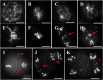
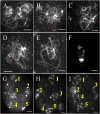
Meiosis is a critical process in sexual reproduction, and errors during this cell division can significantly impact fertility. Successful meiosis relies on the coordinated action of numerous genes involved in DNA replication, strand breaks, and subsequent rejoining. DNA topoisomerase enzymes play a vital role by regulating DNA topology, alleviating tension during replication and transcription. To elucidate the specific function of DNA topoisomerase 1α ( ) in male reproductive development of Arabidopsis thaliana, we investigated meiotic cell division in Arabidopsis flower buds. Combining cytological and biochemical techniques, we aimed to reveal the novel contribution of to meiosis. Our results demonstrate that the absence of leads to aberrant chromatin behavior during meiotic division. Specifically, the top1α1 mutant displayed altered heterochromatin distribution and clustered centromere signals at early meiotic stages. Additionally, this mutant exhibited disruptions in the distribution of 45s rDNA signals and a reduced frequency of chiasma formation during metaphase I, a crucial stage for genetic exchange. Furthermore, the atm-2×top1α1 double mutant displayed even more severe meiotic defects, including incomplete synapsis, DNA fragmentation, and the presence of polyads. These observations collectively suggest that plays a critical role in ensuring accurate meiotic progression, promoting homologous chromosome crossover formation, and potentially functioning in a shared DNA repair pathway with ATAXIA TELANGIECTASIA MUTATED (ATM) in Arabidopsis microspore mother cells.
Keywords: 45s rDNA; ATM; Centromere; DNA topoisomerase 1α; FISH; Meiosis.
© 2024 Elesawi et al.
Conflict of interest statement
Diaa Abd El-Moneim is an Academic Editor for PeerJ.
Figures

References
-
- Armstrong S. Plant Meiosis. Cham: Springer; 2013. Spreading and fluorescence in situ hybridization of male and female meiocyte chromosomes from Arabidopsis thaliana for cytogenetical analysis; pp. 3–11. - PubMed
-
- Chang S, Li C, Jiang Y, Long Y, Li Y, Yin J. Characteristics of the pollen morphology and viability of Bougainvillea (Nyctaginaceae) Scientia Horticulturae. 2021;277:109732. doi: 10.1016/j.scienta.2020.109732. - DOI
-
- Chang F, Zhang Z, Jin Y, Ma H. Flower Development. Cham: Springer; 2014. Cell biological analyses of anther morphogenesis and pollen viability in arabidopsis and rice; pp. 203–216. - PubMed
-
- Cui DL, Xu CX, Wang P, Gao TY, Wang B, Yu TY. Male gametogenesis in flowering plants. Frontiers in Sustainable Food Systems. 2024;7:1333544. doi: 10.3389/fsufs.2023.1333544. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

