A Systematic Review and Meta-Analysis of Eltrombopag Efficacy Combined With Immunosuppressive Drugs in Treatment of Severe Aplastic Anemia
- PMID: 39221321
- PMCID: PMC11365712
- DOI: 10.7759/cureus.65970
A Systematic Review and Meta-Analysis of Eltrombopag Efficacy Combined With Immunosuppressive Drugs in Treatment of Severe Aplastic Anemia
Abstract
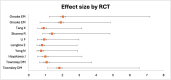
Severe aplastic anemia (SAA) is a life-threatening disorder with high mortality. The only curative treatment is hematopoietic stem cell transplantation (HSCT), but it is mainly for young patients with suitable donors. The alternative is immunosuppressive therapy (IST), which can improve blood counts in about 58% of patients, but many relapse after discontinuation. Recently, eltrombopag, a thrombopoietic receptor agonist, was tested. As a single drug, it improved blood counts in 40-50% of patients. However, combining eltrombopag and IST proved more effective and safer. A review of 20 randomized controlled trials with 2,469 patients showed that the group receiving eltrombopag and IST had a significantly higher overall response rate (86% vs. 74%) after six months. After two years, 54% of the experimental group had relapsed compared to 39% in the control group. Despite this, eltrombopag tends to increase relapse rates over time. In conclusion, combining eltrombopag with IST is a superior treatment for SAA.
Keywords: aplastic anemia; eltrombopag; hematopoietic stem cell transplantation; immunosuppressive therapy; thrombopoietin receptor agonist.
Copyright © 2024, Illango et al.
Conflict of interest statement
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures


References
-
- Narrative review of aplastic anemia-the importance of supportive treatment. Urbanowicz I, Nahaczewska W, Celuch B. Ann Palliat Med. 2021;10:694–699. - PubMed
-
- A systematic review and meta-analysis of the safety and efficacy of anti-thymocyte globulin combined with eltrombopag in the treatment of severe aplastic anemia. Zhang J, Wu Y, Liu J, Han S, Chen L, Wang H, Peng Y. Ann Palliat Med. 2021;10:5549–5560. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
