Serial Changes in Ventricular Strain in Symptomatic Obstructive Hypertrophic Cardiomyopathy Treated With Mavacamten: Insights From the VALOR-HCM Trial
- PMID: 39221824
- PMCID: PMC11410149
- DOI: 10.1161/CIRCIMAGING.124.017185
Serial Changes in Ventricular Strain in Symptomatic Obstructive Hypertrophic Cardiomyopathy Treated With Mavacamten: Insights From the VALOR-HCM Trial
Abstract
Background: In severely symptomatic patients with obstructive hypertrophic cardiomyopathy, VALOR-HCM (A Study to Evaluate Mavacamten in Adults With Symptomatic Obstructive Hypertrophic Cardiomyopathy Who Are Eligible for Septal Reduction Therapy) demonstrated that mavacamten reduces the need for septal reduction therapy with sustained improvement in left ventricular (LV) outflow tract gradients and symptoms. Global longitudinal strain (GLS), a measure of regional myocardial function, is a more sensitive marker of systolic function. In VALOR-HCM, we assessed serial changes in LV and right ventricular (RV) strain.
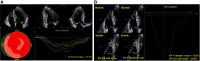
Methods: VALOR-HCM included 112 patients with symptomatic obstructive hypertrophic cardiomyopathy (mean, 60 years; 51% male; LV ejection fraction, 68%). Patients assigned to mavacamten at baseline continued the drug for 56 weeks (n=56) and those assigned to placebo (n=52) transitioned to mavacamten from weeks 16 to 56 (40-week exposure). LV-GLS and RV-GLS assessment was performed using a vendor-neutral software. Non-foreshortened apical (4-, 3-, and 2-chamber) views were used to obtain peak LV-GLS. RV focused 4-chamber view was used to calculate RV 4-chamber and free wall strain. A more negative strain value is favorable.
Results: At baseline, the mean LV-GLS, RV 4-chamber, and free wall strain values were -14.7%, -22.2%, and -16.8%, respectively (all worse than reported normal means). In the total study sample, LV-GLS significantly improved from baseline to week 56 (P=0.02). Twelve patients had transient reduction in LV ejection fraction (<50%) requiring temporary drug interruption (including 3 permanent discontinuations). The LV-GLS in this subgroup was worse at baseline versus total study population (-11.4%), with no significant worsening from baseline through week 56 (P=0.64). Both free wall and 4-chamber RV-GLS remained unchanged from baseline to week 56 (P=0.62 and P=0.56, respectively).
Conclusions: In VALOR-HCM, treatment with mavacamten improved LV-GLS from baseline through week 56 (with no significant worsening of LV-GLS in patients with a reduction in LV ejection fraction ≤50%), suggesting a favorable long-term impact on regional LV systolic function. Additionally, there was no detrimental impact on RV systolic function.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT04349072.
Keywords: Mavacamten; cardiomyopathy, hypertrophic obstructive; global longitudinal strain.
Conflict of interest statement
Dr Desai reports consulting for Bristol Myers Squibb, Cytokinetics, Tenaya, Edgewise, and viz.AI and research support to Cleveland Clinic from Bristol Myers Squibb, Cytokinetics, and Tenaya. Dr Owens reports consulting for Bristol Myers Squibb, Cytokinetics, Pfizer, Biomarin, Tenaya, Lexicon, Stealth, Edgewise, and Renovacor and grant support for research from BMS. Dr Saberi reports consulting for Bristol Myers Squibb and Cytokinetics. Dr Lakdawala has received consulting incomes from Bristol Myers Squibb, Pfizer, Tenaya, Cytokinetics, and Akros and research support from Bristol Myers Squibb and Pfizer. Dr Wang reports research grants (to institution) from Bristol Myers Squibb, Cytokinetics, and Abbott Vascular; being on the consulting/advisory board from Bristol Myers Squibb; being on the steering committee for Bristol Myers Squibb and Cytokinetics; and speaker fees from Bristol Myers Squibb. Drs Naidu, Sherrid, and Tower-Rader report consulting for Bristol Myers Squibb and Cytokinetics. Dr Geske reports consultation with Bristol Myers Squibb. Dr Fermin reports conflicts from Bristol Myers Squibb (consulting, speaking), Pfizer (consulting), and BridgeBio (consulting, speaking). Drs Gaballa, Cremer, Popovic, and Yokushi report no conflicts of interest. Dr Nissen and Wang work for C5 Research and are employees of Cleveland Clinic, which received payments for current research from Bristol Myers Squibb. Drs Lampl and Sehnert are employed by and have stock ownership at Bristol Myers Squibb.
Figures


References
-
- Maron BJ, Desai MY, Nishimura RA, Spirito P, Rakowski H, Towbin JA, Dearani JA, Rowin EJ, Maron MS, Sherrid MV. Management of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79:390–414. doi: 10.1016/j.jacc.2021.11.021 - PubMed
-
- Arbelo E, Protonotarios A, Gimeno JR, Arbustini E, Barriales-Villa R, Basso C, Bezzina CR, Biagini E, Blom NA, de Boer RA, et al. ; ESC Scientific Document Group. 2023 ESC guidelines for the management of cardiomyopathies. Eur Heart J. 2023;44:3503–3626. doi: 10.1093/eurheartj/ehad194 - PubMed
-
- Ommen SR, Ho CY, Asif IM, Balaji S, Burke MA, Day SM, Dearani JA, Epps KC, Evanovich L, Ferrari VA, et al. . 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR guideline for the management of hypertrophic cardiomyopathy: a report of the American Heart Association/American College of Cardiology joint committee on clinical practice guidelines. Circulation. 2024;149:e1239–e1311. doi: 10.1161/CIR.0000000000001250 - PubMed
-
- Ommen SR, Maron BJ, Olivotto I, Maron MS, Cecchi F, Betocchi S, Gersh BJ, Ackerman MJ, McCully RB, Dearani JA, et al. . Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46:470–476. doi: 10.1016/j.jacc.2005.02.090 - PubMed
-
- Smedira NG, Lytle BW, Lever HM, Rajeswaran J, Krishnaswamy G, Kaple RK, Dolney DO, Blackstone EH. Current effectiveness and risks of isolated septal myectomy for hypertrophic obstructive cardiomyopathy. Ann Thorac Surg. 2008;85:127–133. doi: 10.1016/j.athoracsur.2007.07.063 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

