Perioperative toripalimab plus neoadjuvant chemotherapy might improve outcomes in resectable esophageal cancer: an interim analysis of a phase III randomized clinical trial
- PMID: 39221992
- PMCID: PMC11483553
- DOI: 10.1002/cac2.12604
Perioperative toripalimab plus neoadjuvant chemotherapy might improve outcomes in resectable esophageal cancer: an interim analysis of a phase III randomized clinical trial
Abstract
Background: In the era of immunotherapy, neoadjuvant immunochemotherapy (NAIC) for the treatment of locally advanced esophageal squamous cell carcinoma (ESCC) is used clinically but lacks of high-level clinical evidence. This study aimed to compare the safety and long-term efficacy of NAIC followed by minimally invasive esophagectomy (MIE) with those of neoadjuvant chemotherapy (NAC) followed by MIE.
Methods: A prospective, single-center, open-label, randomized phase III clinical trial was conducted at Henan Cancer Hospital, Zhengzhou, China. Patients were randomly assigned to receive either neoadjuvant toripalimab (240 mg) plus paclitaxel (175 mg/m2) + cisplatin (75 mg/m2) (toripalimab group) or paclitaxel + cisplatin alone (chemotherapy group) every 3 weeks for 2 cycles. After surgery, the toripalimab group received toripalimab (240 mg every 3 weeks for up to 6 months). The primary endpoint was event-free survival (EFS). The pathological complete response (pCR) and overall survival (OS) were key secondary endpoints. Adverse events (AEs) and quality of life were also assessed.
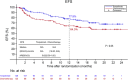
Results: Between May 15, 2020 and August 13, 2021, 252 ESCC patients ranging from T1N1-3M0 to T2-3N0-3M0 were enrolled for interim analysis, with 127 in the toripalimab group and 125 in the chemotherapy group. The 1-year EFS rate was 77.9% in the toripalimab group compared to 64.3% in the chemotherapy group (hazard ratio [HR] = 0.62; 95% confidence interval [CI] = 0.39 to 1.00; P = 0.05). The 1-year OS rates were 94.1% and 83.0% in the toripalimab and chemotherapy groups, respectively (HR = 0.48; 95% CI = 0.24 to 0.97; P = 0.037). The patients in the toripalimab group had a higher pCR rate (18.6% vs. 4.6%; P = 0.001). The rates of postoperative Clavien-Dindo grade IIIb or higher morbidity were 9.8% in the toripalimab group and 6.8% in the chemotherapy group, with no significant difference observed (P = 0.460). The rates of grade 3 or 4 treatment-related AEs did not differ between the two groups (12.5% versus 12.4%).
Conclusions: The interim results of this ongoing trial showed that in resectable ESCC, the addition of perioperative toripalimab to NAC is safe, may improve OS and might change the standard treatment in the future.
Keywords: esophageal squamous cell carcinoma; minimally invasive esophagectomy; neoadjuvant chemotherapy; neoadjuvant immunochemotherapy; survival.
© 2024 The Author(s). Cancer Communications published by John Wiley & Sons Australia, Ltd on behalf of Sun Yat‐sen University Cancer Center.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Demarest CT, Chang AC. The Landmark Series: Multimodal Therapy for Esophageal Cancer. Ann Surg Oncol. 2021;28(6):3375‐82. - PubMed
-
- Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5‐fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19(1):68‐74. - PubMed
-
- Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long‐term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27(30):5062‐7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2022/Central Plains Young Top Talent
- SBGJ202102059/Henan Province Medical Science and Technology Key Projects Coconstructed by the Ministry of Health
- 320.6750.2020-15-1/Wu Jieping Medical Foundation
- YXKC2021029/Henan Province Health Science and Technology Innovation Outstanding Young Talent Training Project
- 82002521/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical

