Impact of Microparticle Transarterial Chemoembolization (mTACE) on myeloid-derived suppressor cell subtypes in hepatocellular carcinoma: Clinical correlations and therapeutic implications
- PMID: 39222024
- PMCID: PMC11367920
- DOI: 10.1002/iid3.70007
Impact of Microparticle Transarterial Chemoembolization (mTACE) on myeloid-derived suppressor cell subtypes in hepatocellular carcinoma: Clinical correlations and therapeutic implications
Abstract
Background: Myeloid-derived suppressor cells (MDSCs) play a pivotal role in immunosuppression and tumor progression in hepatocellular carcinoma (HCC). While various treatments like surgical resection, ablation, and radiotherapy have been studied for their effects on circulating MDSC frequencies in HCC patients, the findings remain inconclusive. Transarterial Chemoembolization (TACE) stands as the standard care for unresectable HCC, with Microparticle TACE (mTACE) gaining prominence for its capacity to induce significant tumor necrosis. However, the immunological ramifications of such pathological outcomes are scarcely reported.
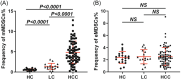
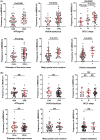
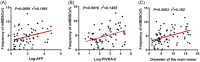
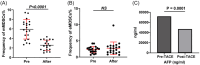
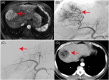
Methods and results: This study aims to elucidate the alterations in MDSC subtypes, specifically monocytic MDSCs (mMDSCs) and early-stage MDSCs (eMDSCs), post-mTACE and to investigate their clinical correlations in HCC patients. A cohort comprising 75 HCC patients, 16 liver cirrhosis patients, and 20 healthy controls (HC) was studied. Peripheral blood samples were collected and analyzed for MDSC subtypes. The study also explored the associations between MDSC frequencies and various clinical parameters in HCC patients. The frequency of mMDSCs was significantly elevated in the HCC group compared to liver cirrhosis and HC. Importantly, mMDSC levels were strongly correlated with aggressive clinical features of HCC, including tumor size, vascular invasion, and distant metastasis. Post-mTACE, a marked reduction in mMDSC frequencies was observed, while eMDSC levels remained stable.
Conclusions: Our findings underscore the critical role of mMDSCs in HCC pathogenesis and their potential as a therapeutic target. The study also highlights the efficacy of mTACE in modulating the immunosuppressive tumor microenvironment, thereby opening new avenues for combinatorial immunotherapeutic strategies in HCC management.
Keywords: Transarterial Chemoembolization (TACE); early stage MDSCs; gelatin sponge microparticles (GSMs); hepatocellular carcinoma (HCC); monocytic‐myeloid‐derived suppressor cells (mMDSCs).
© 2024 The Author(s). Immunity, Inflammation and Disease published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. - PubMed
-
- Elwan N, Salem ML, Kobtan A, et al. High numbers of myeloid derived suppressor cells in peripheral blood and ascitic fluid of cirrhotic and HCC patients. Immunol Invest. 2018;47(2):169‐180. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

