The pro-drug C13 activates AMPK by two distinct mechanisms
- PMID: 39222030
- PMCID: PMC11555695
- DOI: 10.1042/BCJ20240425
The pro-drug C13 activates AMPK by two distinct mechanisms
Abstract
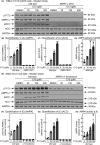
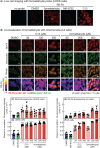
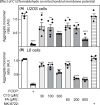
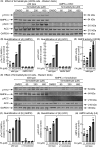
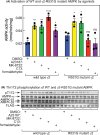
The AMP-activated protein kinase (AMPK) is a sensor of cellular energy status that is expressed in almost all eukaryotic cells. In the canonical activation mechanism, it is activated by increases in AMP:ATP and ADP:ATP ratios that signify declining cellular energy status. Once activated, AMPK phosphorylates numerous targets that promote catabolic pathways generating ATP, while inhibiting anabolic and other processes that consume ATP, thus acting to restore energy homeostasis. Pharmacological agents that activate AMPK have been useful in identifying downstream targets and have potential as drugs for treatment of metabolic disorders such as Type 2 diabetes and non-alcoholic fatty liver disease. One such agent is C13, a pro-drug with a phosphonate bis(isobutyryloxymethyl) ester moiety, with the isobutyryloxymethyl groups increasing membrane permeability. Following cellular uptake, C13 is cleaved to release C2, an AMP analogue and potent AMPK activator that is specific for complexes containing the α1 (but not the α2) catalytic subunit isoform. This has previously been assumed to be the sole mechanism by which C13 activates AMPK, with potential roles for the isobutyryloxymethyl groups being ignored. We now report that, following cleavage from C13, these protective groups are metabolized to formaldehyde, an agent that inhibits mitochondrial function and increases cellular AMP:ATP ratios, thus providing additional AMPK activation by the canonical mechanism.
Keywords: AMPK; C13; formaldehyde; mitochondria; mitochondrial respiration.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

