Hepatocellular carcinoma and AIM2: Therapeutic potential through regulation of autophagy and macrophage polarization
- PMID: 39222064
- PMCID: PMC11367919
- DOI: 10.1002/iid3.70002
Hepatocellular carcinoma and AIM2: Therapeutic potential through regulation of autophagy and macrophage polarization
Abstract
Objective: Hepatocellular carcinoma (HCC) poses a significant challenge to global health. Its pathophysiology involves interconnected processes, including cell proliferation, autophagy, and macrophage polarization. However, the role of Absent in Melanoma 2 (AIM2) in HCC remains elusive.
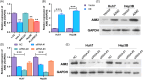
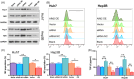
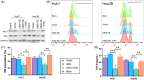
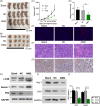
Methods: The expression of AIM2 in Huh-7 and Hep3B cell lines was manipulated and cell proliferation, autophagy, apoptosis, and migration/invasion, together with the polarization of M2 macrophages, were evaluated. The markers of autophagy pathway, LC3B, Beclin-1, and P62, underwent examination through Western blot analysis. An autophagy inhibitor, 3-MA, was used to measured the role of autophagy in HCC. Finally, the effect of AIM2 overexpression on HCC was further evaluated using a subcutaneous tumor model in nude mice.
Results: Our results established that AIM2 overexpression inhibits HCC cell proliferation, migration, and invasion while promoting apoptosis and autophagy. Conversely, knockdown of AIM2 engendered opposite effects. AIM2 overexpression was correlated with reduced M2 macrophage polarization. The autophagy inhibitor substantiated AIM2's role in autophagy and identified its downstream impact on cell proliferation, migration, invasion, and macrophage polarization. In the in vivo model, overexpression of AIM2 led to the inhibition of HCC tumor growth.
Conclusion: The findings underscore AIM2's crucial function in modulating major biological processes in HCC, pointing to its potential as a therapeutic target. This study inaugurally demonstrated that AIM2 activates autophagy and influences macrophage polarization, playing a role in liver cancer progression.
Keywords: Absent in Melanoma 2; autophagy; cell proliferation; hepatocellular carcinoma; macrophage polarization.
© 2024 The Author(s). Immunity, Inflammation and Disease published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Zheng HC, Zhou J, Chen YC, et al. The burden and trend of liver metastases in shanghai, China: a population‐based study. Eur J Cancer Prev. 2023;32:517‐524. - PubMed
-
- Peng C, Ye Z, Ju Y, et al. Mechanism of action and treatment of type I interferon in hepatocellular carcinoma. Clin Transl Oncol. 2023;26:326‐337. - PubMed
-
- Chen YH, Tsai CH, Chen YY, et al. The efficacy and safety of ramucirumab in heavily pretreated advanced hepatocellular carcinoma. Anticancer Res. 2023;43:3203‐3212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

