Acetyl-DL-leucine in two individuals with REM sleep behavior disorder improves symptoms, reverses loss of striatal dopamine-transporter binding and stabilizes pathological metabolic brain pattern-case reports
- PMID: 39223119
- PMCID: PMC11369233
- DOI: 10.1038/s41467-024-51502-7
Acetyl-DL-leucine in two individuals with REM sleep behavior disorder improves symptoms, reverses loss of striatal dopamine-transporter binding and stabilizes pathological metabolic brain pattern-case reports
Abstract
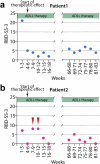
Isolated REM Sleep Behavior Disorder (iRBD) is considered a prodrome of Parkinson's disease (PD). We investigate whether the potentially disease-modifying compound acetyl-DL-leucine (ADLL; 5 g/d) has an effect on prodromal PD progression in 2 iRBD-patients. Outcome parameters are RBD-severity sum-score (RBD-SS-3), dopamine-transporter single-photon emission computerized tomography (DAT-SPECT) and metabolic "Parkinson-Disease-related-Pattern (PDRP)"-z-score in 18F-fluorodeoxyglucose positron emission tomography (FDG-PET). After 3 weeks ADLL-treatment, the RBD-SS-3 drops markedly in both patients and remains reduced for >18 months of ADLL-treatment. In patient 1 (female), the DAT-SPECT putaminal binding ratio (PBR) decreases in the 5 years pretreatment from normal (1.88) to pathological (1.22) and the patient's FDG-PET-PDRP-z-score rises from 1.72 to 3.28 (pathological). After 22 months of ADLL-treatment, the DAT-SPECT-PBR increases to 1.67 and the FDG-PET-PDRP-z-score stabilizes at 3.18. Similar results are seen in patient 2 (male): his DAT-SPECT-PBR rises from a pretreatment value of 1.42 to 1.72 (close to normal) and the FDG-PET-PDRP-z-score decreases from 1.02 to 0.30 after 18 months of ADLL-treatment. These results support exploration of whether ADLL may have disease-modifying properties in prodromal PD.
© 2024. The Author(s).
Conflict of interest statement
Wolfgang H. Oertel has received speaker’s honoria on educational symposia sponsored by Abbvie, the International Movement Disorders Society and Stada Pharma. He acts as a consultant for Lario Therapeutics and is a member of advisory boards with Intrabio and MODAG. He holds stock options with Intrabio related to this manuscript and stock options with MODAG unrelated to this work. The institution of W.H.O., not W.H.O personally received/s scientific grants from the German Research Foundation, the Michael J Fox Foundation and Rittal Foundation unrelated to the manuscript. Jan Booij is a consultant of GE Healthcare. The institution of J.B., not J.B. personally received research funding from GE Healthcare. Lars Timmermann has received speaker’s honoria on educational symposia sponsored by Abbvie, Boston Scientific, DIAPLAN, Neuraxpharm, Novartis, the International Movement Disorders Society und Teva. He has been a consultant for Boston Scientific. The institution of L.T., not L.T. personally received/s funding by Boston Scientific, the German Research Foundation, the German Ministry of Education and Research, the Otto-Loewi-Foundation and the Deutsche Parkinson Vereinigung. Neither L.T. nor any member of his family holds stocks, stock options, patents or financial interests in any of the above-mentioned companies or their competitors. Michael Strupp is Joint Chief Editor of the Journal of Neurology, Editor in Chief of Frontiers of Neuro-otology and Section Editor of F1000. He has received speaker’s honoraria on educational symposia sponsored by Abbott, Auris Medical, Biogen, Eisai, Grünenthal, GSK, Henning Pharma, Interacoustics, J&J, MSD, NeuroUpdate, Otometrics, Pierre-Fabre, TEVA, UCB, and Viatris. He acts as a consultant for Abbott, AurisMedical, Bulbitec, Heel, IntraBio, Sensorion and Vertify. He is an investor and share-holder of IntraBio. The institution of M.S., not M.S. personally received/s support for clinical studies from Decibel, USA, Cure within Reach, USA and Heel, Germany. He distributes “M-glasses” and “Positional vertigo App”. The remaining authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

