Neurovascular coupling and CO2 interrogate distinct vascular regulations
- PMID: 39223128
- PMCID: PMC11369082
- DOI: 10.1038/s41467-024-49698-9
Neurovascular coupling and CO2 interrogate distinct vascular regulations
Abstract
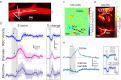
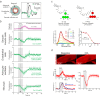
Neurovascular coupling (NVC), which mediates rapid increases in cerebral blood flow in response to neuronal activation, is commonly used to map brain activation or dysfunction. Here we tested the reemerging hypothesis that CO2 generated by neuronal metabolism contributes to NVC. We combined functional ultrasound and two-photon imaging in the mouse barrel cortex to specifically examine the onsets of local changes in vessel diameter, blood flow dynamics, vascular/perivascular/intracellular pH, and intracellular calcium signals along the vascular arbor in response to a short and strong CO2 challenge (10 s, 20%) and whisker stimulation. We report that the brief hypercapnia reversibly acidifies all cells of the arteriole wall and the periarteriolar space 3-4 s prior to the arteriole dilation. During this prolonged lag period, NVC triggered by whisker stimulation is not affected by the acidification of the entire neurovascular unit. As it also persists under condition of continuous inflow of CO2, we conclude that CO2 is not involved in NVC.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
- EQU201903007811/Fondation pour la Recherche Médicale (Foundation for Medical Research in France)
- NR-16-RHUS-0004 [RHU TRT_cSVD]/Agence Nationale de la Recherche (French National Research Agency)
- 16CVD05/Fondation Leducq
- ANR-18-IAHU-0001/Agence Nationale de la Recherche (French National Research Agency)
- SPF201909009103/Fondation pour la Recherche Médicale (Foundation for Medical Research in France)
LinkOut - more resources
Full Text Sources
Research Materials

