Gut microbiota patterns associated with duration of diarrhea in children under five years of age in Ethiopia
- PMID: 39223134
- PMCID: PMC11369280
- DOI: 10.1038/s41467-024-51464-w
Gut microbiota patterns associated with duration of diarrhea in children under five years of age in Ethiopia
Abstract
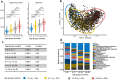
Diarrhea claims >500,000 lives annually among children under five years of age in low- and middle-income countries. Mortality due to acute diarrhea (<7 days' duration) is decreasing, but prolonged (7-13 days) and persistent (≥14 days of duration) diarrhea remains a massive challenge. Here, we use a case-control study to decipher if fecal gut microbiota compositional differences between Ethiopian children with acute (n=554) or prolonged/persistent (n=95) diarrhea and frequency-matched non-diarrheal controls (n=663) are linked to diarrheal etiology. We show that diarrhea cases are associated with lower bacterial diversity and enriched in Escherichia spp., Campylobacter spp., and Streptococcus spp. Further, diarrhea cases are depleted in gut commensals such as Prevotella copri, Faecalibacterium prausnitzii, and Dialister succinatiphilus, with depletion being most pronounced in prolonged/persistent cases, suggesting that prolonged duration of diarrhea is accompanied by depletion of gut commensals and that re-establishing these via e.g., microbiota-directed food supplements offer a potential treatment strategy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

