Induction of oxidative- and endoplasmic-reticulum-stress dependent apoptosis in pancreatic cancer cell lines by DDOST knockdown
- PMID: 39223141
- PMCID: PMC11369111
- DOI: 10.1038/s41598-024-68510-8
Induction of oxidative- and endoplasmic-reticulum-stress dependent apoptosis in pancreatic cancer cell lines by DDOST knockdown
Abstract
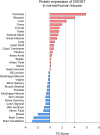
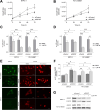
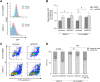
The dolichyl-diphosphooligosaccharide-protein glycosyltransferase non-catalytic subunit (DDOST) is a key component of the oligosaccharyltransferase complex catalyzing N-linked glycosylation in the endoplasmic reticulum lumen. DDOST is associated with several cancers and congenital disorders of glycosylation. However, its role in pancreatic cancer remains elusive, despite its enriched pancreatic expression. Using quantitative mass spectrometry, we identify 30 differentially expressed proteins and phosphopeptides (DEPs) after DDOST knockdown in the pancreatic ductal adenocarcinoma (PDAC) cell line PA-TU-8988T. We evaluated DDOST / DEP protein-protein interaction networks using STRING database, correlation of mRNA levels in pancreatic cancer TCGA data, and biological processes annotated to DEPs in Gene Ontology database. The inferred DDOST regulated phenotypes were experimentally verified in two PDAC cell lines, PA-TU-8988T and BXPC-3. We found decreased proliferation and cell viability after DDOST knockdown, whereas ER-stress, ROS-formation and apoptosis were increased. In conclusion, our results support an oncogenic role of DDOST in PDAC by intercepting cell stress events and thereby reducing apoptosis. As such, DDOST might be a potential biomarker and therapeutic target for PDAC.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

