Cancer drug-tolerant persister cells: from biological questions to clinical opportunities
- PMID: 39223250
- PMCID: PMC12622869
- DOI: 10.1038/s41568-024-00737-z
Cancer drug-tolerant persister cells: from biological questions to clinical opportunities
Abstract
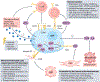
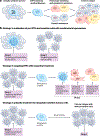
The emergence of drug resistance is the most substantial challenge to the effectiveness of anticancer therapies. Orthogonal approaches have revealed that a subset of cells, known as drug-tolerant 'persister' (DTP) cells, have a prominent role in drug resistance. Although long recognized in bacterial populations which have acquired resistance to antibiotics, the presence of DTPs in various cancer types has come to light only in the past two decades, yet several aspects of their biology remain enigmatic. Here, we delve into the biological characteristics of DTPs and explore potential strategies for tracking and targeting them. Recent findings suggest that DTPs exhibit remarkable plasticity, being capable of transitioning between different cellular states, resulting in distinct DTP phenotypes within a single tumour. However, defining the biological features of DTPs has been challenging, partly due to the complex interplay between clonal dynamics and tissue-specific factors influencing their phenotype. Moreover, the interactions between DTPs and the tumour microenvironment, including their potential to evade immune surveillance, remain to be discovered. Finally, the mechanisms underlying DTP-derived drug resistance and their correlation with clinical outcomes remain poorly understood. This Roadmap aims to provide a comprehensive overview of the field of DTPs, encompassing past achievements and current endeavours in elucidating their biology. We also discuss the prospect of future advancements in technologies in helping to unveil the features of DTPs and propose novel therapeutic strategies that could lead to their eradication.
© 2024. Springer Nature Limited.
Conflict of interest statement
Competing interests
A.B. reports receipt of grants/research supports from Neophore, AstraZeneca and Boehringer Ingelheim and honoraria/consultation fees from Guardant Health and Inivata. A.B. is a stock shareholder of Neophore and Kither Biotech. A.B. is an advisory boards member for Inivata, Neophore and Roche/Genentech. E.B. provides consultancy services to Roche, and his laboratory has entered into sponsored research agreements and received funding from Merus NL, Incyte and Revolution Medicines. S.S. reports personal fees from Agence nationale de la recherche (France), Krebsliga Schweiz (Switzerland), KWF Kankerbestrijding (The Netherlands) and Shenzhen Medical Academy of Research and Translation (China). M.J.G. has received research grants from AstraZeneca, GlaxoSmithKline and Astex Pharmaceuticals and is a consultant for and holds equity in Mosaic Therapeutics. M.H. is a cofounder and consultant for, and has received research grant funding from, Ferro Therapeutics (BridgeBio). M.R., M.C., E.M., H.P., S.K.R., E.S., A.S., T.S.T., N.Q.B., R.B., E.L., J.-C.M., C.A.O., Y.O., E.E.P., C.R. and S.M.R. declare no competing interests.
Figures

References
-
-
Sharma SV et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell 141, 69–80 (2010).
The emergence of DTP cells following drug treatment of cancer cells was described for the first time in this landmark study, which also highlighted the selective sensitivity of DTP cells to inhibition of the KDM5 epigenetic enzyme.
-
-
- Shen S, Vagner S & Robert C Persistent cancer cells: the deadly survivors. Cell 183, 860–874 (2020). - PubMed
-
- Marine JC, Dawson SJ & Dawson MA Non-genetic mechanisms of therapeutic resistance in cancer. Nat. Rev. Cancer 20, 743–756 (2020). - PubMed
-
- Bigger J Treatment of staphylococcal infections with penicillin by intermittent sterilisation. Lancet 244, 497–500 (1944).
-
- Hobby GL, Meyer K & Chaffee E Observations on the mechanism of action of penicillin. Proc. Soc. Exp. Biol. Med. 50, 281–285 (1942).

