Tumour-derived small extracellular vesicles act as a barrier to therapeutic nanoparticle delivery
- PMID: 39223270
- PMCID: PMC11838174
- DOI: 10.1038/s41563-024-01961-6
Tumour-derived small extracellular vesicles act as a barrier to therapeutic nanoparticle delivery
Abstract
Nanoparticles are promising for drug delivery applications, with several clinically approved products. However, attaining high nanoparticle accumulation in solid tumours remains challenging. Here we show that tumour cell-derived small extracellular vesicles (sEVs) block nanoparticle delivery to tumours, unveiling another barrier to nanoparticle-based tumour therapy. Tumour cells secrete large amounts of sEVs in the tumour microenvironment, which then bind to nanoparticles entering tumour tissue and traffic them to liver Kupffer cells for degradation. Knockdown of Rab27a, a gene that controls sEV secretion, decreases sEV levels and improves nanoparticle accumulation in tumour tissue. The therapeutic efficacy of messenger RNAs encoding tumour suppressing and proinflammatory proteins is greatly improved when co-encapsulated with Rab27a small interfering RNA in lipid nanoparticles. Together, our results demonstrate that tumour cell-derived sEVs act as a defence system against nanoparticle tumour delivery and that this system may be a potential target for improving nanoparticle-based tumour therapies.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests: N.G., M.J.M., W.Z. and W.G. have filed a patent (Lipid nanoparticle (LNP) compositions and methods for delivering therapeutic agents to tumour cells) related to this paper.
Figures
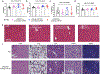

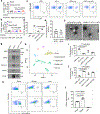
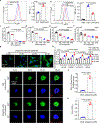
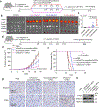


References
-
- D’Mello SR et al. The evolving landscape of drug products containing nanomaterials in the United States. Nat. Nanotech. 12, 523–529 (2017). - PubMed
-
- Sindhwani S et al. The entry of nanoparticles into solid tumours. Nat. Mater. 19, 566–575 (2020). - PubMed
-
- Wilhelm S et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 1, 1–12 (2016).
-
- Sykes EA, Chen J, Zheng G & Chan WC Investigating the impact of nanoparticle size on active and passive tumor targeting efficiency. ACS nano 8, 5696–5706 (2014). - PubMed
Methods-only references.
-
- Ohara Y et al. Effective delivery of chemotherapeutic nanoparticles by depleting host Kupffer cells. Int. J. Cancer 131, 2402–2410 (2012). - PubMed
-
- Gong N et al. In situ PEGylation of CAR T cells alleviates cytokine release syndrome and neurotoxicity. Nat. Mater, 1–10 (2023). - PubMed
-
- Huo S et al. Superior Penetration and Retention Behavior of 50 nm Gold Nanoparticles in TumorsCell Penetration and Tumor Retention of Gold Nanoparticles. Cancer Res. 73, 319–330 (2013). - PubMed
-
- Xing L, Zheng H, Cao Y & Che S Coordination polymer coated mesoporous silica nanoparticles for pH-responsive drug release. Adv. Mater. 24, 6433–6437 (2012). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

