Deficient RPE mitochondrial energetics leads to subretinal fibrosis in age-related neovascular macular degeneration
- PMID: 39223298
- PMCID: PMC11369096
- DOI: 10.1038/s42003-024-06773-7
Deficient RPE mitochondrial energetics leads to subretinal fibrosis in age-related neovascular macular degeneration
Abstract
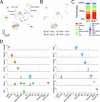
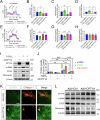
Subretinal fibrosis permanently impairs the vision of patients with neovascular age-related macular degeneration. Despite emerging evidence revealing the association between disturbed metabolism in retinal pigment epithelium (RPE) and subretinal fibrosis, the underlying mechanism remains unclear. In the present study, single-cell RNA sequencing revealed, prior to subretinal fibrosis, genes in mitochondrial fatty acid oxidation are downregulated in the RPE lacking very low-density lipoprotein receptor (VLDLR), especially the rate-limiting enzyme carnitine palmitoyltransferase 1A (CPT1A). We found that overexpression of CPT1A in the RPE of Vldlr-/- mice suppresses epithelial-to-mesenchymal transition and fibrosis. Mechanistically, TGFβ2 induces fibrosis by activating a Warburg-like effect, i.e. increased glycolysis and decreased mitochondrial respiration through ERK-dependent CPT1A degradation. Moreover, VLDLR blocks the formation of the TGFβ receptor I/II complex by interacting with unglycosylated TGFβ receptor II. In conclusion, VLDLR suppresses fibrosis by attenuating TGFβ2-induced metabolic reprogramming, and CPT1A is a potential target for treating subretinal fibrosis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
- EY033330/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- R01 EY034742/EY/NEI NIH HHS/United States
- EY019309/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- EY034510/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- R01 EY028949/EY/NEI NIH HHS/United States
- EY034742/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- EY032930/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- R01 EY032931/EY/NEI NIH HHS/United States
- R01 EY032930/EY/NEI NIH HHS/United States
- R01 EY028773/EY/NEI NIH HHS/United States
- R01 EY033477/EY/NEI NIH HHS/United States
- EY033477/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- R01 EY034510/EY/NEI NIH HHS/United States
- EY028949/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- R01 EY033330/EY/NEI NIH HHS/United States
- EY032931/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- R01 EY019309/EY/NEI NIH HHS/United States
- EY028773/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

