Mitochondria transfer-based therapies reduce the morbidity and mortality of Leigh syndrome
- PMID: 39223312
- PMCID: PMC12188917
- DOI: 10.1038/s42255-024-01125-5
Mitochondria transfer-based therapies reduce the morbidity and mortality of Leigh syndrome
Abstract
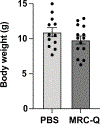
Mitochondria transfer is a recently described phenomenon in which donor cells deliver mitochondria to acceptor cells1-3. One possible consequence of mitochondria transfer is energetic support of neighbouring cells; for example, exogenous healthy mitochondria can rescue cell-intrinsic defects in mitochondrial metabolism in cultured ρ0 cells or Ndufs4-/- peritoneal macrophages4-7. Exposing haematopoietic stem cells to purified mitochondria before autologous haematopoietic stem cell transplantation allowed for treatment of anaemia in patients with large-scale mitochondrial DNA mutations8,9, and mitochondria transplantation was shown to minimize ischaemic damage to the heart10-12, brain13-15 and limbs16. However, the therapeutic potential of using mitochondria transfer-based therapies to treat inherited mitochondrial diseases is unclear. Here we demonstrate improved morbidity and mortality of the Ndufs4-/- mouse model of Leigh syndrome (LS) in multiple treatment paradigms associated with mitochondria transfer. Transplantation of bone marrow from wild-type mice, which is associated with release of haematopoietic cell-derived extracellular mitochondria into circulation and transfer of mitochondria to host cells in multiple organs, ameliorates LS in mice. Furthermore, administering isolated mitochondria from wild-type mice extends lifespan, improves neurological function and increases energy expenditure of Ndufs4-/- mice, whereas mitochondria from Ndufs4-/- mice did not improve neurological function. Finally, we demonstrate that cross-species administration of human mitochondria to Ndufs4-/- mice also improves LS. These data suggest that mitochondria transfer-related approaches can be harnessed to treat mitochondrial diseases, such as LS.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Figures



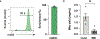
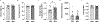


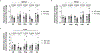


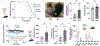
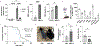
References
MeSH terms
Substances
Grants and funding
- 21K08415/MEXT | Japan Society for the Promotion of Science (JSPS)
- JPMJSP #2138/MEXT | Japan Science and Technology Agency (JST)
- 22K16322/MEXT | Japan Society for the Promotion of Science (JSPS)
- 20K17379/MEXT | Japan Society for the Promotion of Science (JSPS)
- R01 NS134932/NS/NINDS NIH HHS/United States
- U54 NS078059/NS/NINDS NIH HHS/United States
- 24PRE1189775/American Heart Association (American Heart Association, Inc.)
- 5U54-NS078059-12/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- 1R01NS134932/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- CAMS #1019648/Burroughs Wellcome Fund (BWF)
- 24POST1244220/American Heart Association (American Heart Association, Inc.)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

