Innate immune sensing of cell death in disease and therapeutics
- PMID: 39223376
- PMCID: PMC12459733
- DOI: 10.1038/s41556-024-01491-y
Innate immune sensing of cell death in disease and therapeutics
Abstract
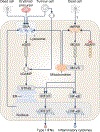
Innate immunity, cell death and inflammation underpin many aspects of health and disease. Upon sensing pathogens, pathogen-associated molecular patterns or damage-associated molecular patterns, the innate immune system activates lytic, inflammatory cell death, such as pyroptosis and PANoptosis. These genetically defined, regulated cell death pathways not only contribute to the host defence against infectious disease, but also promote pathological manifestations leading to cancer and inflammatory diseases. Our understanding of the underlying mechanisms has grown rapidly in recent years. However, how dying cells, cell corpses and their liberated cytokines, chemokines and inflammatory signalling molecules are further sensed by innate immune cells, and their contribution to further amplify inflammation, trigger antigen presentation and activate adaptive immunity, is less clear. Here, we discuss how pattern-recognition and PANoptosome sensors in innate immune cells recognize and respond to cell-death signatures. We also highlight molecular targets of the innate immune response for potential therapeutic development.
© 2024. Springer Nature Limited.
Conflict of interest statement
Competing financial interests
T-D.K. and S.M.M. have no interests to declare.
Figures

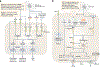

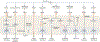
References
-
- Brennan MA & Cookson BT Salmonella induces macrophage death by caspase-1-dependent necrosis. Molecular microbiology 38, 31–40 (2000). - PubMed
-
- Kayagaki N et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011). - PubMed
-
- Kayagaki N et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015). - PubMed
-
- Shi J et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R35 CA253095/CA/NCI NIH HHS/United States
- AR056296/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 AI101935/AI/NIAID NIH HHS/United States
- AI124346/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 AI124346/AI/NIAID NIH HHS/United States
- Ideas Grant APP2002686/Department of Health | National Health and Medical Research Council (NHMRC)
- R37 AI101935/AI/NIAID NIH HHS/United States
- R01 AI160179/AI/NIAID NIH HHS/United States
- AI160179/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- N/A/American Lebanese Syrian Associated Charities (ALSAC)
- Investigator Grant 2026910/Department of Health | National Health and Medical Research Council (NHMRC)
- AI101935/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 AR056296/AR/NIAMS NIH HHS/United States
- CA253095/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
LinkOut - more resources
Full Text Sources
Medical

