A review of methods for the analysis of diagnostic tests performed in sequence
- PMID: 39223640
- PMCID: PMC11370044
- DOI: 10.1186/s41512-024-00175-3
A review of methods for the analysis of diagnostic tests performed in sequence
Abstract
Background: Many clinical pathways for the diagnosis of disease are based on diagnostic tests that are performed in sequence. The performance of the full diagnostic sequence is dictated by the diagnostic performance of each test in the sequence as well as the conditional dependence between them, given true disease status. Resulting estimates of performance, such as the sensitivity and specificity of the test sequence, are key parameters in health-economic evaluations. We conducted a methodological review of statistical methods for assessing the performance of diagnostic tests performed in sequence, with the aim of guiding data analysts towards classes of methods that may be suitable given the design and objectives of the testing sequence.
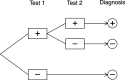
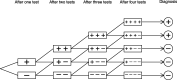
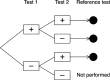
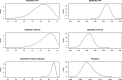
Methods: We searched PubMed, Scopus and Web of Science for relevant papers describing methodology for analysing sequences of diagnostic tests. Papers were classified by the characteristics of the method used, and these were used to group methods into themes. We illustrate some of the methods using data from a cohort study of repeat faecal immunochemical testing for colorectal cancer in symptomatic patients, to highlight the importance of allowing for conditional dependence in test sequences and adjustment for an imperfect reference standard.
Results: Five overall themes were identified, detailing methods for combining multiple tests in sequence, estimating conditional dependence, analysing sequences of diagnostic tests used for risk assessment, analysing test sequences in conjunction with an imperfect or incomplete reference standard, and meta-analysis of test sequences.
Conclusions: This methodological review can be used to help researchers identify suitable analytic methods for studies that use diagnostic tests performed in sequence.
Keywords: Conditional dependence; Diagnosis; Diagnostic accuracy; Sequential diagnostic testing.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
