Bile acids impact the microbiota, host, and C. difficile dynamics providing insight into mechanisms of efficacy of FMTs and microbiota-focused therapeutics
- PMID: 39224076
- PMCID: PMC11376424
- DOI: 10.1080/19490976.2024.2393766
Bile acids impact the microbiota, host, and C. difficile dynamics providing insight into mechanisms of efficacy of FMTs and microbiota-focused therapeutics
Erratum in
-
Correction.Gut Microbes. 2024 Jan-Dec;16(1):2411134. doi: 10.1080/19490976.2024.2411134. Epub 2024 Oct 3. Gut Microbes. 2024. PMID: 39361787 Free PMC article. No abstract available.
Abstract
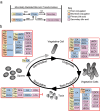
Clostridioides difficile is a major nosocomial pathogen, causing significant morbidity and mortality worldwide. Antibiotic usage, a major risk factor for Clostridioides difficile infection (CDI), disrupts the gut microbiota, allowing C. difficile to proliferate and cause infection, and can often lead to recurrent CDI (rCDI). Fecal microbiota transplantation (FMT) and live biotherapeutic products (LBPs) have emerged as effective treatments for rCDI and aim to restore colonization resistance provided by a healthy gut microbiota. However, much is still unknown about the mechanisms mediating their success. Bile acids, extensively modified by gut microbes, affect C. difficile's germination, growth, and toxin production while also shaping the gut microbiota and influencing host immune responses. Additionally, microbial interactions, such as nutrient competition and cross-feeding, contribute to colonization resistance against C. difficile and may contribute to the success of microbiota-focused therapeutics. Bile acids as well as other microbial mediated interactions could have implications for other diseases being treated with microbiota-focused therapeutics. This review focuses on the intricate interplay between bile acid modifications, microbial ecology, and host responses with a focus on C. difficile, hoping to shed light on how to move forward with the development of new microbiota mediated therapeutic strategies to combat rCDI and other intestinal diseases.
Keywords: Clostridioides difficile; bile acids; fecal microbiota transplantation; microbiota; nuclear receptors; recurrent CDI.
Conflict of interest statement
C.M.T. consults for Vedanta Bioscicens, Inc., Summit Therapeutics, and Ferring Pharmaceuticals, Inc. and is on the Scientic Advisory Board for Ancilia Biosciences.
Figures
References
-
- CDC . Antibiotic resistance threats in the United States, 2019. Atlanta (GA): U.S. Department of Health and Human Services, CDC; 2019.
-
- Theriot CM, Koenigsknecht MJ, Carlson PE, Hatton GE, Nelson AM, Li B, Huffnagle GB, Z. Li, J., Young VB. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to clostridium difficile infection. Nat Commun. 2014;5(1):3114. doi: 10.1038/ncomms4114. - DOI - PMC - PubMed
-
- Johnson S, Lavergne V, Skinner AM, Gonzales-Luna AJ, Garey KW, Kelly CP, Wilcox MH. Clinical practice guideline by the infectious diseases society of America (IDSA) and society for healthcare epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin Infect Dis. 2021;73(5):e1029–e1044. doi: 10.1093/cid/ciab549. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
