O,S,Se-containing Biginelli products based on cyclic β-ketosulfone and their postfunctionalization
- PMID: 39224228
- PMCID: PMC11368051
- DOI: 10.3762/bjoc.20.184
O,S,Se-containing Biginelli products based on cyclic β-ketosulfone and their postfunctionalization
Abstract
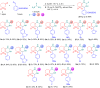
A one-pot three-component Biginelli synthesis of dihydropyrimidinones/thiones/selenones via acetic acid or solvent-free Yb(OTf)3-catalyzed tandem reaction of β-ketosulfone (dihydro-2H-thiopyran-3(4H)-one-1,1-dioxide), an appropriate urea, and arylaldehyde has been developed. The reaction proceeds with high chemo- and regioselectivity to give diverse DHPMs in reasonable yields up to 95%. Moreover, an SO2-containing analogue of anticancer drug-candidate enastron (SO2 vs C=O) was obtained by using the here reported method in gram scale. We also demonstrate the reactivity of the Biginelli product in various directions - synthesis of condensed thiazoles and tetrazoles. In silico assessment of ADMET parameters shows that most compounds meet the lead-likeness requirements. The biological profiles of new compounds demonstrate high probability levels of activity against the following pathogens/diseases: Candida albicans, Alphis gossypii, Tripomastigote Chagas, Tcruzi amastigota, Tcruzi epimastigota, Leishmania amazonensis, and Dengue larvicida.
Keywords: dihydropyrimidinone/thione/selenone; green chemistry; in silico biological profile; multicomponent reaction (MCR); thiopyrandioxide.
Copyright © 2024, Dil and Palchykov.
Figures


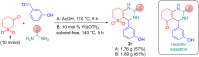


References
-
- Faizan S, Roohi T F, Raju R M, Sivamani Y, BR P K. J Mol Struct. 2023;1291:136020. doi: 10.1016/j.molstruc.2023.136020. - DOI
-
- Chopda L V, Dave P N. ChemistrySelect. 2020;5:5552–5572. doi: 10.1002/slct.202000742. - DOI
-
- Costanzo P, Nardi M, Oliverio M. Eur J Org Chem. 2020:3954–3964. doi: 10.1002/ejoc.201901923. - DOI
LinkOut - more resources
Full Text Sources
