MicroRNA dysregulation in ataxia telangiectasia
- PMID: 39224604
- PMCID: PMC11366618
- DOI: 10.3389/fimmu.2024.1444130
MicroRNA dysregulation in ataxia telangiectasia
Abstract
Introduction: Ataxia telangiectasia (AT) is a rare disorder characterized by neurodegeneration, combined immunodeficiency, a predisposition to malignancies, and high clinical variability. Profiling of microRNAs (miRNAs) may offer insights into the underlying mechanisms of complex rare human diseases, as miRNAs play a role in various biological functions including proliferation, differentiation, and DNA repair. In this study, we investigate the differential expression of miRNAs in samples from AT patients to identify miRNA patterns and analyze how these patterns are related to the disease.
Methods: We enrolled 20 AT patients (mean age 17.7 ± 9.6 years old) and collected clinical and genetic data. We performed short non-coding RNA-seq analysis on peripheral blood mononuclear cells (PBMCs) and fibroblasts to compare the miRNA expression profile between AT patients and controls.
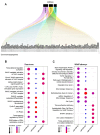
Results: We observed 42 differentially expressed (DE)-miRNAs in blood samples and 26 in fibroblast samples. Among these, three DE-miRNAs, miR-342-3p, miR-30a-5p, and miR-195-5p, were further validated in additional AT samples, confirming their dysregulation.
Discussion: We identified an AT-related miRNA signature in blood cells and fibroblast samples collected from a group of AT patients. We also predicted several dysregulated pathways, primarily related to cancer, immune system control, or inflammatory processes. The findings suggest that miRNAs may provide insights into the pathophysiology and tumorigenesis of AT and have the potential to serve as useful biomarkers in cancer research.
Keywords: DNA repair; ataxia telangiectasia; cancer; immunodeficiency; microRNA.
Copyright © 2024 Cirillo, Tarallo, Toriello, Carissimo, Giardino, De Rosa, Damiano, Soresina, Badolato, Dellepiane, Baselli, Carrabba, Fabio, Bertolini, Montin, Conti, Romano, Pozzi, Ferrero, Roncarati, Ferracin, Brusco, Parenti and Pignata.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

