Ferulic Acid Methyl Ester Attenuates Cerebral Ischemia-Reperfusion Injury in Rats by Modulating PI3K/HIF-1α/VEGF Signaling Pathway
- PMID: 39224659
- PMCID: PMC11368119
- DOI: 10.2147/JIR.S473665
Ferulic Acid Methyl Ester Attenuates Cerebral Ischemia-Reperfusion Injury in Rats by Modulating PI3K/HIF-1α/VEGF Signaling Pathway
Abstract
Background: Cerebral ischaemia-reperfusion injury (CIRI) could worsen the inflammatory response and oxidative stress in brain tissue. According to previous studies, ferulic acid methyl ester (FAME), as the extract with the strongest comprehensive activity in the traditional Chinese medicine Huang Hua oil dot herb, has significant anti-oxidative stress and neuroprotective functions, and can effectively alleviate CIRI, but its mechanism of action is still unclear.
Methods: Firstly, the pharmacological effects of FAME were investigated by in vitro oxidative stress and inflammatory experiments. Secondly, evaluate the therapeutic effects of FAME in the treatment of CIRI by brain histopathological staining and cerebral infarct area by replicating the in vivo MACO model. Thirdly, RNA-Seq and network pharmacology were utilized to predict the possible targets and mechanisms of FAME for CIRI at the molecular level. Finally, the expression of key target proteins, as well as the key regulatory relationships were verified by molecular docking visualization, Western Blotting and immunohistochemistry.
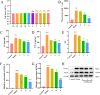
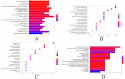
Results: The results of in vitro experiments concluded that FAME could significantly reduce the content of TNF-α, IL-1β and ROS, inhibiting COX-2 and iNOS protein expression in cells(p<0.01). FAME was demonstrated to have anti-oxidative stress and anti-inflammatory effects. The results of in vivo experiments showed that after the administration of FAME, the area of cerebral infarction in rats with CIRI was reduced, the content of Bcl-2 and VEGF was increased(p<0.05). Network pharmacology and RNA-Seq showed that the alleviation of CIRI by FAME may be through PI3K-AKT and HIF-1 signaling pathway. Enhanced expression of HIF-1α, VEGF, p-PI3K, p-AKT proteins in the brain tissues of rats in the FAME group was verified by molecular docking and Western Blotting.
Conclusion: FAME possesses significant anti-inflammatory and anti-oxidative stress activities and alleviates CIRI through the PI3K/HIF-1α/VEGF signaling pathway.
Keywords: RNA-Seq; WGCNA; cerebral ischemia-reperfusion injury; ferulic acid methyl ester; molecular docking techniques; network pharmacology.
© 2024 Zhou et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures










References
-
- Jurcau A, Ardelean IA. Molecular pathophysiological mechanisms of ischemia/reperfusion injuries after recanalization therapy for acute ischemic stroke. J Integr Neurosci. 2021;20:727–744. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

