Leveraging a global, federated, real-world data network to optimize investigator-initiated pediatric clinical trials: the TriNetX Pediatric Collaboratory Network
- PMID: 39224867
- PMCID: PMC11368118
- DOI: 10.1093/jamiaopen/ooae077
Leveraging a global, federated, real-world data network to optimize investigator-initiated pediatric clinical trials: the TriNetX Pediatric Collaboratory Network
Abstract
Objective: Clinical research networks facilitate collaborative research, but data sharing remains a common barrier.
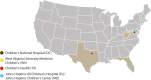
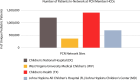
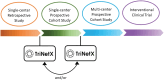
Materials and methods: The TriNetX platform provides real-time access to electronic health record (EHR)-derived, anonymized data from 173 healthcare organizations (HCOs) and tools for queries and analysis. In 2022, 4 pediatric HCOs worked with TriNetX leadership to found the Pediatric Collaboratory Network (PCN), facilitated via a multi-institutional data-use agreement (DUA). The DUA enables collaborative study design and execution, with institutional review board-approved transfer of complete datasets for further analyses on a per-protocol basis.
Results and discussion: Of the 41.2 million children with TriNetX records, the PCN represents nearly 10%. The PCN assisted several early-career investigators to bring study concepts from conception to an international scientific meeting presentation and journal submission.
Conclusion: The PCN facilitates EHR vendor-agnostic multicenter pediatric research on the global TriNetX platform. Continued growth of the PCN will advance knowledge in pediatric health.
Keywords: electronic health record; pediatric; real-world data.
© The Author(s) 2024. Published by Oxford University Press on behalf of the American Medical Informatics Association.
Conflict of interest statement
A.Z. has received honoraria from Sanofi and Takeda for her role as an advisory board member for scientific advisory boards in the past 3 years. N.A.G. has received or has recently received consultancy fees from Anthos Therapeutics, Bayer, Boehringer-Ingelheim, Daiichi Sankyo, and the University of Colorado-affiliated Academic Research Organization CPC Clinical Research for roles in clinical trial planning or oversight committees (eg, advisory committee; steering committee; data and safety monitoring committee) in pharmaceutical industry-sponsored pediatric clinical trials of antithrombotics. All other authors have indicated they have no financial relationships or potential conflicts of interest relevant to this article to disclose.
Figures
References
-
- Institute of Medicine (US) Forum on Drug Discovery, Development and Translation . Transforming clinical research in the United States: challenges and opportunities: workshop summary. In: The National Academies Collection: Reports Funded by National Institutes of Health. Washington, DC: National Academies Press; 2010. - PubMed
-
- Frei E, Holland JF, Schneiderman MA, et al. A comparative study of two regimens of combination chemotherapy in acute leukemia. Blood. 1958;13(12):1126-1148. - PubMed
-
- Brunner HI, Rider LG, Kingsbury DJ, PRCSG Advisory Council, et al. Pediatric Rheumatology Collaborative Study Group—over four decades of pivotal clinical drug research in pediatric rheumatology. Pediatr Rheumatol Online J. 2018;16(1):45. 10.1186/s12969-018-0261-x[published Online First: 20180711]. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
