Peptidic "Molecular Beacon" for Collagen
- PMID: 39225003
- PMCID: PMC11563731
- DOI: 10.1021/acs.biomac.4c01000
Peptidic "Molecular Beacon" for Collagen
Abstract
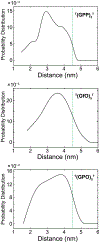
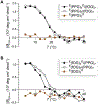
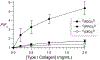
Collagen-mimetic peptides (CMP) have been invaluable tools for understanding the structure and function of collagen, which is the most abundant protein in animals. CMPs have also been developed as probes that detect damaged collagen because of the specificity required to form a collagen triple helix. These probes are not, however, ratiometric. Here, we used EPR spectroscopy to determine the end-to-end distances of CMPs that do not form stable homotrimeric helices. We found that those distances are shorter than the distances in the context of a collagen triple helix, suggesting their potential utility as a "molecular beacon" and guiding the choice and location of a pendant fluorophore-quencher pair. We then showed that a molecular beacon based on a glycine-(2S,4S)-4-fluoroproline-(2S,4R)-4-hydroxyproline tripeptide repeat and EDANS-DABCYL pair enabled the ratiometric detection of its binding to both other CMPs and natural mammalian collagen. These results provide guidance for the development of a new modality for detecting damaged collagen in physiological settings.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Fratzl P, Collagen: Structure and Mechanics. Springer, Boston, MA: 2008.
-
- Bella J Collagen structure: New tricks from a very old dog. Biochem. J 2016, 473, 1001–1025. - PubMed
-
- Ramshaw JAM; Shah NK; Brodsky B Gly–X–Y tripeptide frequencies in collagen: A context for host–guest triple-helical peptides. J. Struct. Biol 1998, 122, 86–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

