Pharmacologic LDH inhibition redirects intratumoral glucose uptake and improves antitumor immunity in solid tumor models
- PMID: 39225102
- PMCID: PMC11364391
- DOI: 10.1172/JCI177606
Pharmacologic LDH inhibition redirects intratumoral glucose uptake and improves antitumor immunity in solid tumor models
Abstract
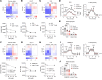
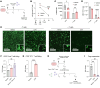
Tumor reliance on glycolysis is a hallmark of cancer. Immunotherapy is more effective in controlling glycolysis-low tumors lacking lactate dehydrogenase (LDH) due to reduced tumor lactate efflux and enhanced glucose availability within the tumor microenvironment (TME). LDH inhibitors (LDHi) reduce glucose uptake and tumor growth in preclinical models, but their impact on tumor-infiltrating T cells is not fully elucidated. Tumor cells have higher basal LDH expression and glycolysis levels compared with infiltrating T cells, creating a therapeutic opportunity for tumor-specific targeting of glycolysis. We demonstrate that LDHi treatment (a) decreases tumor cell glucose uptake, expression of the glucose transporter GLUT1, and tumor cell proliferation while (b) increasing glucose uptake, GLUT1 expression, and proliferation of tumor-infiltrating T cells. Accordingly, increasing glucose availability in the microenvironment via LDH inhibition leads to improved tumor-killing T cell function and impaired Treg immunosuppressive activity in vitro. Moreover, combining LDH inhibition with immune checkpoint blockade therapy effectively controls murine melanoma and colon cancer progression by promoting effector T cell infiltration and activation while destabilizing Tregs. Our results establish LDH inhibition as an effective strategy for rebalancing glucose availability for T cells within the TME, which can enhance T cell function and antitumor immunity.
Keywords: Cancer immunotherapy; Glucose metabolism; Immunology; Metabolism; Pharmacology.
Conflict of interest statement
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

