Enhanced Photocatalytic Properties and Photoinduced Crystallization of TiO2-Fe2O3 Inverse Opals Fabricated by Atomic Layer Deposition
- PMID: 39225124
- PMCID: PMC11403546
- DOI: 10.1021/acsami.4c10831
Enhanced Photocatalytic Properties and Photoinduced Crystallization of TiO2-Fe2O3 Inverse Opals Fabricated by Atomic Layer Deposition
Abstract
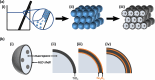
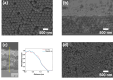
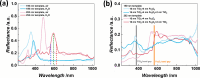
The use of solar energy for photocatalysis holds great potential for sustainable pollution reduction. Titanium dioxide (TiO2) is a benchmark material, effective under ultraviolet light but limited in visible light utilization, restricting its application in solar-driven photocatalysis. Previous studies have shown that semiconductor heterojunctions and nanostructuring can broaden the TiO2's photocatalytic spectral range. Semiconductor heterojunctions are interfaces formed between two different semiconductor materials that can be engineered. Especially, type II heterojunctions facilitate charge separation, and they can be obtained by combining TiO2 with, for example, iron(III) oxide (Fe2O3). Nanostructuring in the form of 3D inverse opals (IOs) demonstrated increased TiO2 light absorption efficiency of the material, by tailoring light-matter interactions through their photonic crystal structure and specifically their photonic stopband, which can give rise to a slow photon effect. Such effect is hypothesized to enhance the generation of free charges. This work focuses on the above-described effects simultaneously, through the synthesis of TiO2-Fe2O3 IOs via multilayer atomic layer deposition (ALD) and the characterization of their photocatalytic activities. Our results reveal that the complete functionalization of TiO2 IOs with Fe2O3 increases the photocatalytic activity through the slow photon effect and semiconductor heterojunction formation. We systematically explore the influence of Fe2O3 thickness on photocatalytic performance, and a maximum photocatalytic rate constant of 1.38 ± 0.09 h-1 is observed for a 252 nm template TiO2-Fe2O3 bilayer IO consisting of 16 nm TiO2 and 2 nm Fe2O3. Further tailoring the performance by overcoating with additional TiO2 layers enhances photoinduced crystallization and tunes photocatalytic properties. These findings highlight the potential of TiO2-Fe2O3 IOs for efficient water pollutant removal and the importance of precise nanostructuring and heterojunction engineering in advancing photocatalytic technologies.
Keywords: atomic layer deposition; inverse opal; multilayer thin films; photocatalysis; photoinduced crystallization; semiconductor heterostructure.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Kumar N.; Kumbhat S.. Essentials in Nanoscience and Nanotechnology; John Wiley & Sons Inc.: Hoboken, NJ, 2016.
-
- Carp O.; Huisman C. L.; Reller A. Photoinduced Reactivity of Titanium Dioxide. Prog. Solid State Chem. 2004, 32 (1–2), 33–177. 10.1016/j.progsolidstchem.2004.08.001. - DOI
Publication types
LinkOut - more resources
Full Text Sources

