In vitro exposure to benzo[a]pyrene damages the developing mouse ovary
- PMID: 39225137
- PMCID: PMC10160542
- DOI: 10.1530/RAF-22-0071
In vitro exposure to benzo[a]pyrene damages the developing mouse ovary
Abstract
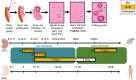
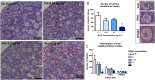
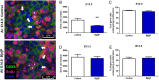
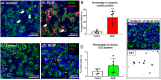
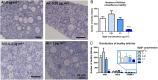
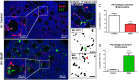
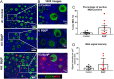
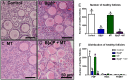
Females are born with a finite number of oocytes, collectively termed the ovarian reserve, established within the developing fetal ovary. Consequently, maternal exposure to reproductive toxicants can have harmful effects on the future fertility of her unborn female fetus. The chemical benzo[a]pyrene (B[a]P) is a prominent component of cigarette smoke. Despite it being a known ovotoxicant, around 8% of women in Europe smoke during pregnancy. The purpose of this research was to examine the effect of B[a]P on the developing ovary, using the mouse as a model and with experiments carried out in vitro. B[a]P-exposure to the fetal ovary prior to follicle formation reduced the number of germ cells and subsequently, the number of healthy primordial follicles, by up to 76%; however, while proliferation of germ cells was not affected, the germ cells contained higher levels of DNA double-strand breaks. Exposure to B[a]P also affected the proportion of oocytes progressing through prophase I of meiosis. B[a]P exposure to neonatal mouse ovaries, after follicle formation, resulted in an 85% reduction in the number of healthy follicles, with a corresponding increase in apoptotic cell death and reduction in somatic cell proliferation. Although there was a trend towards a higher level of oxidative stress in B[a]P-exposed ovaries, this was not statistically significant; likewise, the antioxidant melatonin failed to protect against the B[a]P-induced ovarian damage. Together, the results here demonstrate that B[a]P-exposure damages the developing ovary, both before and shortly after follicle formation, an effect that could lead to a subsequent decrease in fertility.
This work is licensed under a Creative Commons Attribution 4.0 International License.
Conflict of interest statement
Norah Spears is Co-Editor-in-Chief of Reproduction and Fertility and was therefore not involved in the review or editorial process associated with this paper of which she is a co-author.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

