Novel Tubeimoside I liposomal drug delivery system in combination with gemcitabine for the treatment of pancreatic cancer
- PMID: 39225145
- PMCID: PMC11485868
- DOI: 10.1080/17435889.2024.2382076
Novel Tubeimoside I liposomal drug delivery system in combination with gemcitabine for the treatment of pancreatic cancer
Abstract
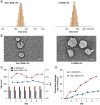
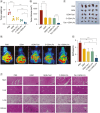
Aim: To evaluate the anti-pancreatic cancer effect of novel Tubeimoside I multifunctional liposomes combined with gemcitabine.Methods: Liposomes were prepared through the thin film hydration method, with evaluations conducted on parameters including encapsulation efficiency (EE%), particle size, polydispersity index (PDI), zeta potential (ZP), storage stability, and release over a 7-day period. The cellular uptake rate, therapeutic efficacy in vitro and in vivo and the role of immune microenvironment modulation were evaluated.Results: The novel Tubeimoside I multifunctional liposomal exhibited good stability, significant anti-cancer activity, and immune microenvironment remodeling effects. Furthermore, it showed a safety profile.Conclusion: This study underscores the potential of Novel Tubeimoside I multifunctional liposomal as a promising treatment option for pancreatic cancer.
Keywords: F18-FDG micro-PET/CT imaging; gemcitabine; liposomes; pancreatic cancer; tubular alfalfa glycosides I; tumor microenvironment.
Plain language summary
[Box: see text].
Conflict of interest statement
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Figures






Similar articles
-
Carrier-Free Nanomedicine Based on Celastrol and Methotrexate for Synergistic Treatment of Breast Cancer via Folate Targeting.Int J Nanomedicine. 2025 Jun 27;20:8291-8304. doi: 10.2147/IJN.S516921. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40599402 Free PMC article.
-
Biomimetic cancer cell membrane-enriched vitamin E-stapled gemcitabine-loaded TPGS micelles for pancreatic cancer therapy.Drug Deliv. 2025 Dec;32(1):2527759. doi: 10.1080/10717544.2025.2527759. Epub 2025 Jul 9. Drug Deliv. 2025. PMID: 40631432 Free PMC article.
-
Self-targeted liposomes for enhancing chemotherapeutic efficacy of pancreatic cancer by degrading the extracellular matrix and eradicating intra-tumoral bacteria.Biomater Sci. 2025 Jul 22;13(15):4123-4138. doi: 10.1039/d5bm00786k. Biomater Sci. 2025. PMID: 40522076
-
An Updated Meta-analysis and System Review:is Gemcitabine+Fluoropyrimidine in Combination a Better Therapy Versus Gemcitabine Alone for Advanced and Unresectable Pancreatic Cancer?Asian Pac J Cancer Prev. 2015;16(14):5681-6. doi: 10.7314/apjcp.2015.16.14.5681. Asian Pac J Cancer Prev. 2015. PMID: 26320435
-
Efficacy and safety of gemcitabine plus erlotinib for locally advanced or metastatic pancreatic cancer: a systematic review and meta-analysis.Drug Des Devel Ther. 2016 Jun 13;10:1961-72. doi: 10.2147/DDDT.S105442. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 27358556 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
