Simultaneously Controlling Inflammation and Infection by Smart Nanomedicine Responding to the Inflammatory Microenvironment
- PMID: 39225387
- PMCID: PMC11497003
- DOI: 10.1002/advs.202403934
Simultaneously Controlling Inflammation and Infection by Smart Nanomedicine Responding to the Inflammatory Microenvironment
Abstract
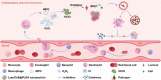
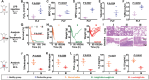
The overactivated immune cells in the infectious lesion may lead to irreversible organ damages under severe infections. However, clinically used immunosuppressive anti-inflammatory drugs will usually disturb immune homeostasis and conversely increase the risk of infections. Regulating the balance between anti-inflammation and anti-infection is thus critical in treating certain infectious diseases. Herein, considering that hydrogen peroxide (H2O2), myeloperoxidase (MPO), and neutrophils are upregulated in the inflammatory microenvironment and closely related to the severity of appendectomy patients, an inflammatory-microenvironment-responsive nanomedicine is designed by using poly(lactic-co-glycolic) acid (PLGA) nanoparticles to load chlorine E6 (Ce6), a photosensitizer, and luminal (Lum), a chemiluminescent agent. The obtained Lum/Ce6@PLGA nanoparticles, being non-toxic within normal physiological environment, can generate cytotoxic single oxygen via bioluminescence resonance energy transfer (BRET) in the inflammatory microenvironment with upregulated H2O2 and MPO, simultaneously killing pathogens and excessive inflammatory immune cells in the lesion, without disturbing immune homeostasis. As evidenced in various clinically relevant bacterial infection models and virus-induced pneumonia, Lum/Ce6@PLGA nanoparticles appeared to be rather effective in controlling both infection and inflammation, resulting in significantly improved animal survival. Therefore, the BRET-based nanoparticles by simultaneously controlling infections and inflammation may be promising nano-therapeutics for treatment of severe infectious diseases.
Keywords: anti‐infection and anti‐inflammation treatment; bioluminescence resonance energy transfer; inflammatory microenvironment; myeloperoxidase; neutrophils.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Torres A., Cilloniz C., Niederman M. S., Menéndez R., Chalmers J. D., Wunderink R. G., van der Poll T., Nat. Rev. Dis. Primers 2021, 7, 25. - PubMed
-
- Saavedra P. H. V., Lamkanfi M., Nature 2017, 546, 606. - PubMed
-
- Nathan C., Cars O., N. Engl. J. Med. 2014, 371, 1761. - PubMed
-
- Holmes A. H., Moore L. S. P., Sundsfjord A., Steinbakk M., Regmi S., Karkey A., Guerin P. J., Piddock L. J. V., Lancet 2016, 387, 176. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
