Association Between SARS-CoV-2 Viral Load and COVID-19 Vaccination in 4 Phase 3 Trials
- PMID: 39225478
- PMCID: PMC11646576
- DOI: 10.1093/infdis/jiae400
Association Between SARS-CoV-2 Viral Load and COVID-19 Vaccination in 4 Phase 3 Trials
Abstract
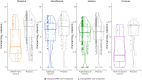
Coronavirus disease 2019 (COVID-19) vaccines reduce severe disease and mortality and may lessen transmission, measured by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral load (VL). Evaluating vaccine associations in VL at COVID-19 diagnosis in 4 phase 3 randomized, placebo-controlled vaccine trials, July 2020 to July 2021, VL reductions were 2.78 log10 copies/mL (95% confidence interval [CI], 1.38-4.18; n = 60 placebo, 11 vaccine) and 2.12 log10 copies/mL (95% CI, 1.44-2.80; n = 594 placebo, 36 vaccine) for NVX-CoV2373 and mRNA-1273, respectively. Associations were not significant for AZD1222 (0.59 log10 copies/mL; 95% CI, -.19 to 1.36; n = 90 placebo, 78 vaccine) or Ad26.COV2.S (0.23 log10 copies/mL; 95% CI, -.01 to .47; n = 916 placebo, 424 vaccine). Thus, vaccines potentially decreased transmission when ancestral SARS-CoV-2 predominated. Clinical Trials Registration. NCT04470427, NCT04505722, NCT04516746, NCT04611802.
Keywords: COVID-19 transmission; COVID-19 vaccine; SARS-CoV-2 viral load; infectiousness; randomized trial.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. A. F. had/has research grants from Pfizer, Merck, Janssen, CyanVac, VaxCo, Moderna, and BioFire Diagnostics; fees for advisory boards from GSK, ADMA Biologics, and Sanofi Pasteur; and fees for data safety monitoring board from Novavax. M. E. S. had/has grants from the National Institutes of Health (NIH) and National Institute of Allergy and Infectious Diseases (NIAID) during the conduct of the study (5UM1AI069470); grants from NIH/NIAID and Gates Foundation outside the submitted work; and grants to her institution from Merck Sharpe and Dohme, Sanofi, and Gilead outside of the submitted work. S. R. W. has received institutional funding from the NIH/NIAID; institutional grants or contracts from Sanofi Pasteur, Janssen Vaccines/Johnson & Johnson, Moderna Tx, Pfizer, Vir Biotechnology, and Worcester HIV Vaccine; has participated on data safety monitoring or advisory boards for Janssen Vaccines/Johnson & Johnson and BioNTech; and his spouse holds stock/stock options in Regeneron Pharmaceuticals. C. M. E. has received research grants from Merck and AstraZeneca; and has participated in Sanofi, Janssen, and Gilead advisory board meetings. N. R. is a paid safety consultant for ICON, CyanVac, and EMMES; served on selected advisory boards for Sanofi, Seqirus, Pfizer, and Moderna; and receives funds to their institution to conduct research from Sanofi, Lilly, Merck, Quidel, Immorna, Vaccine Company, and Pfizer. C. L. G. has received institutional funding from the NIH for the conduct of the Moderna and Novavax studies; and grants to her institution from Gilead and ViiV outside of the submitted work. K. L. K. has received institutional funding from the NIH for the conduct of the Moderna and Novavax studies (UM1 AI 148689); and from Novavax outside of the submitted work. J. A. G. is employed by and holds stock in AstraZeneca. E. A. H. received fees from Oxford University for time spent adjudicating end points for the AstraZeneca/Oxford SARS-CoV-2 vaccine trials conducted in the UK, South Africa, and Brazil. M. D. T. has received institutional funding from the NIH for the conduct of the Moderna and Novavax studies (UM1 AI 148689). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UM1 AI069476/AI/NIAID NIH HHS/United States
- UM1 AI069470/AI/NIAID NIH HHS/United States
- UM1 AI068614/AI/NIAID NIH HHS/United States
- UM1 AI148573/AI/NIAID NIH HHS/United States
- UM1 AI148684/AI/NIAID NIH HHS/United States
- U01 AI068635/AI/NIAID NIH HHS/United States
- United States Government
- UM1 AI148689/AI/NIAID NIH HHS/United States
- UM1 AI148450/AI/NIAID NIH HHS/United States
- and Development Authority
- U01 AI069470/AI/NIAID NIH HHS/United States
- UM1 AI068635/AI/NIAID NIH HHS/United States
- Biomedical Advanced Research
- U01 AI068614/AI/NIAID NIH HHS/United States
- UM1 AI148574/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

