MYC is Sufficient to Generate Mid-Life High-Grade Serous Ovarian and Uterine Serous Carcinomas in a p53-R270H Mouse Model
- PMID: 39225558
- PMCID: PMC11425777
- DOI: 10.1158/2767-9764.CRC-24-0144
MYC is Sufficient to Generate Mid-Life High-Grade Serous Ovarian and Uterine Serous Carcinomas in a p53-R270H Mouse Model
Abstract
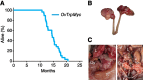
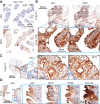
Genetically engineered mouse models (GEMM) have fundamentally changed how ovarian cancer etiology, early detection, and treatment are understood. MYC, an oncogene, is amongst the most amplified genes in high-grade serous ovarian cancer (HGSOC), but it has not previously been utilized to drive HGSOC GEMMs. We coupled Myc and dominant-negative mutant p53-R270H with a fallopian tube epithelium (FTE)-specific promoter Ovgp1 to generate a new GEMM of HGSOC. Female mice developed lethal cancer at an average of 14.5 months. Histopathologic examination of mice revealed HGSOC characteristics, including nuclear p53 and nuclear MYC in clusters of cells within the FTE and ovarian surface epithelium. Unexpectedly, nuclear p53 and MYC clustered cell expression was also identified in the uterine luminal epithelium, possibly from intraepithelial metastasis from the FTE. Extracted tumor cells exhibited strong loss of heterozygosity at the p53 locus, leaving the mutant allele. Copy-number alterations in these cancer cells were prevalent, disrupting a large fraction of genes. Transcriptome profiles most closely matched human HGSOC and serous endometrial cancer. Taken together, these results demonstrate that the Myc and Trp53-R270H transgenes were able to recapitulate many phenotypic hallmarks of HGSOC through the utilization of strictly human-mimetic genetic hallmarks of HGSOC. This new mouse model enables further exploration of ovarian cancer pathogenesis, particularly in the 50% of HGSOC which lack homology-directed repair mutations. Histologic and transcriptomic findings are consistent with the hypothesis that uterine serous cancer may originate from the FTE.
Significance: Mouse models using transgenes which generate spontaneous cancers are essential tools to examine the etiology of human diseases. Here, the first Myc-driven spontaneous model is described as a valid HGSOC model. Surprisingly, aspects of uterine serous carcinoma were also observed in this model.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
G. Fullbright reports grants from the NIH/National Institute of General Medical Sciences during the conduct of the study. R.L. Carpenter reports a patent to 18/418,110 pending. D.T. Long reports grants from the NIH/National Institute of General Medical Sciences during the conduct of the study. J.R. Delaney reports grants from the NIH and Rivkin Foundation during the conduct of the study. No disclosures were reported by the other authors.
Figures




Update of
-
MYC is sufficient to generate mid-life high-grade serous ovarian and uterine serous carcinomas in a p53-R270H mouse model.bioRxiv [Preprint]. 2024 Jan 29:2024.01.24.576924. doi: 10.1101/2024.01.24.576924. bioRxiv. 2024. Update in: Cancer Res Commun. 2024 Sep 1;4(9):2525-2538. doi: 10.1158/2767-9764.CRC-24-0144. PMID: 38352443 Free PMC article. Updated. Preprint.
References
-
- Ferriss JS, Erickson BK, Shih I-M, Fader AN. Uterine serous carcinoma: key advances and novel treatment approaches. Int J Gynecol Cancer 2021;31:1165–74. - PubMed
-
- González-Martín A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 2019;381:2391–402. - PubMed
-
- Mirza MR, Chase DM, Slomovitz BM, dePont Christensen R, Novák Z, Black D, et al. Dostarlimab for primary advanced or recurrent endometrial cancer. N Engl J Med 2023;388:2145–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

