The #HOPE4LIVER Single-Arm Pivotal Trial for Histotripsy of Primary and Metastatic Liver Tumors
- PMID: 39225612
- PMCID: PMC11427859
- DOI: 10.1148/radiol.233051
The #HOPE4LIVER Single-Arm Pivotal Trial for Histotripsy of Primary and Metastatic Liver Tumors
Abstract
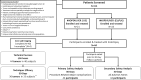
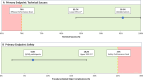
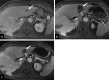
Background Histotripsy is a nonthermal, nonionizing, noninvasive, focused US technique that relies on cavitation for mechanical tissue breakdown at the focal point. Preclinical data have shown its safety and technical success in the ablation of liver tumors. Purpose To evaluate the safety and technical success of histotripsy in destroying primary or metastatic liver tumors. Materials and Methods The parallel United States and European Union and England #HOPE4LIVER trials were prospective, multicenter, single-arm studies. Eligible patients were recruited at 14 sites in Europe and the United States from January 2021 to July 2022. Up to three tumors smaller than 3 cm in size could be treated. CT or MRI and clinic visits were performed at 1 week or less preprocedure, at index-procedure, 36 hours or less postprocedure, and 30 days postprocedure. There were co-primary end points of technical success of tumor treatment and absence of procedure-related major complications within 30 days, with performance goals of greater than 70% and less than 25%, respectively. A two-sided 95% Wilson score CI was derived for each end point. Results Forty-four participants (21 from the United States, 23 from the European Union or England; 22 female participants, 22 male participants; mean age, 64 years ± 12 [SD]) with 49 tumors were enrolled and treated. Eighteen participants (41%) had hepatocellular carcinoma and 26 (59%) had non-hepatocellular carcinoma liver metastases. The maximum pretreatment tumor diameter was 1.5 cm ± 0.6 and the maximum post-histotripsy treatment zone diameter was 3.6 cm ± 1.4. Technical success was observed in 42 of 44 treated tumors (95%; 95% CI: 84, 100) and procedure-related major complications were reported in three of 44 participants (7%; 95% CI: 2, 18), both meeting the performance goal. Conclusion The #HOPE4LIVER trials met the co-primary end-point performance goals for technical success and the absence of procedure-related major complications, supporting early clinical adoption. Clinical trial registration nos. NCT04572633, NCT04573881 Published under a CC BY 4.0 license. Supplemental material is available for this article. See also the editorial by Nezami and Georgiades in this issue.
Conflict of interest statement
Figures

References
-
- Vidal-Jove J , Serres X , Vlaisavljevich E , et al . First-in-man histotripsy of hepatic tumors: the THERESA trial, a feasibility study . Int J Hyperthermia 2022. ; 39 ( 1 ): 1115 – 1123 . - PubMed
-
- Bray F , Ferlay J , Soerjomataram I , Siegel RL , Torre LA , Jemal A . Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries . CA Cancer J Clin 2018. ; 68 ( 6 ): 394 – 424 . [Published correction appears in CA Cancer J Clin 2020;70(4):313.] - PubMed
-
- Heimbach JK , Kulik LM , Finn RS , et al . AASLD guidelines for the treatment of hepatocellular carcinoma . Hepatology 2018. ; 67 ( 1 ): 358 – 380 . - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

