Immune checkpoint inhibitors-associated cranial nerves involvement: a systematic literature review on 136 patients
- PMID: 39225744
- PMCID: PMC11446990
- DOI: 10.1007/s00415-024-12660-2
Immune checkpoint inhibitors-associated cranial nerves involvement: a systematic literature review on 136 patients
Abstract
Objective: Describe the demographic data and clinical phenotype of cranial palsy induced by immune checkpoint inhibitors (CNP-ICI).
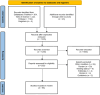
Methods: A systematic literature review of the literature was performed in Pubmed, Web of Science, and Embase, including 68 articles and 136 patients (PROSPERO no. CRD42024517262).
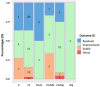
Results: Out of the 1205 articles screened, 68 articles were included after fulfilling the inclusion criteria, for a total of 136 patients. All articles were case reports and case series. In the cohort studied, 52% of patients were treated with anti PD-1/PDL-1 therapies, 14% with anti CTLA-4 therapies, and 34% with a combination of anti CTLA-4 and anti PD-1/PDL-1 therapies. The facial nerve was the most affected cranial nerve, involved in 38% of cases, followed by the optic nerve (35%), the cochleovestibular nerve (12%), and the abducens nerve (10%). The median time from the initial immune checkpoint inhibitor (ICI) injection to the onset CNP-ICI was 10 weeks (IQR 4-20). Magnetic resonance imaging demonstrated contrast enhancement or abnormal signal of the affected nerve in 43% of cases. Cerebrospinal fluid analysis indicated lymphocytic pleocytosis in 59% of cases. At the onset of immune-related adverse events, 89% of patients discontinued immunotherapy, and 92% received treatment for CNP-ICI. Treatment regimens included corticosteroids in 86% of cases, intravenous immunoglobulin in 21%, and plasma exchange in 5.1%. Among the whole population, 33% achieved recovery, 52% showed clinical improvement, 16% remained stable, and 3% experienced worsening of their condition. Rechallenge with immunotherapy was significantly associated with the emergence of new immune-related Adverse Events (irAEs).
Conclusion: ICI therapy may lead to cranial nerve involvement, particularly affecting the facial nerve, typically presenting around 10 weeks after treatment initiation. While corticosteroid therapy often resulted in patient improvement, rechallenging with ICIs were associated with new irAEs.
Keywords: Cranial palsy; Immune checkpoint inhibitors; Immune-related adverse events; Ipilimumab; Nivolumab; Pembrolizumab.
© 2024. The Author(s).
Conflict of interest statement
Authors have no conflict of interest to declare.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

