Three-dimensional printed calcium phosphate scaffolds emulate bone microstructure to promote bone regrowth and repair
- PMID: 39225913
- PMCID: PMC11371849
- DOI: 10.1007/s10856-024-06817-8
Three-dimensional printed calcium phosphate scaffolds emulate bone microstructure to promote bone regrowth and repair
Abstract
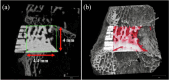
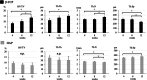
The interconnected structures in a 3D scaffold allows the movement of cells and nutrients. Therefore, this study aimed to investigate the in-vivo bioactivity of 3D-printed β-tricalcium phosphate (β-TCP) and hydroxyapatite (HAP) scaffolds that replicate biological bone. This study included 24-week-old male New Zealand white rabbits. A cylindrical bone defect with a diameter of 4.5 mm and a depth of 8 mm was created in the lateral aspect of the distal femur. A 3D-printed scaffold was implanted in the right femur (experimental side), whereas the left femur was kept free of implantation (control side). Micro-CT analysis and histological observations of the bone defect site were conducted at 4, 8, and 12 weeks postoperatively to track the bone repair progress. No evidence of new bone tissue formation was found in the medullary cavity of the bone defect on the control side. In contrast, on the experimental side, the 3D scaffold demonstrated sufficient bioactivity, leading to the growth of new bone tissue. Over time, new bone tissue gradually extended from the periphery toward the center, a phenomenon evident in both micro-CT images and biopsy staining. In the current study, we observed that the cells involved in bone metabolism adhered, spread, and proliferated on our newly designed 3D-printed scaffold with a bone microstructure. Therefore, it is suggested that this scaffold has sufficient bioactivity to induce new bone formation and could be expected to be a more useful artificial bone than the existing version.
© 2024. The Author(s).
Conflict of interest statement
All β-TCP and HAP scaffolds used in the present study were provided free of charge by Tomita Pharmaceutical Co., Ltd.
Figures



References
-
- Tessier P, Kawamoto H, Matthews D, Posnick J, Raulo Y, Tulasne JF. et al. Autogenous bone grafts and bone substitutes e tools and techniques: I. A 20,000-case experience in maxillofacial and craniofacial surgery. Plast Reconstr Surg. 2005;116:72S–73S. 10.1097/01.prs.0000173841.59063.7e. - PubMed
-
- Myeroff C, Archdeacon M. Autogenous bone graft: donor sites and techniques. J Bone Joint Surg Am. 2011;93:2227–36. 10.2106/JBJS.J.01513. - PubMed
-
- Gul H, Khan M, Khan AS 3. Bioceramics: types and clinical applications. Woodhead Publ. Ser. Biomater. 2020:53–83. 10.1016/B978-0-08-102834-6.00003-3.
-
- Dorozhkin SV. Bioceramics of calcium orthophosphates. Biomaterials. 2010;31:1465–85. 10.1016/j.biomaterials.2009.11.050. - PubMed
-
- Kano S, Yamazaki A, Otsuka R, Ohgaki M, Akao M, Aoki H. Application of hydroxyapatite-sol as drug carrier. Bio Med Mater Eng. 1994;4:283–90. 10.3233/BME-1994-4404. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

