GLP-1 Receptor Agonist Use and Risk of Suicide Death
- PMID: 39226030
- PMCID: PMC11372654
- DOI: 10.1001/jamainternmed.2024.4369
GLP-1 Receptor Agonist Use and Risk of Suicide Death
Erratum in
-
Error in Figure 1.JAMA Intern Med. 2024 Nov 1;184(11):1396. doi: 10.1001/jamainternmed.2024.6163. JAMA Intern Med. 2024. PMID: 39495245 Free PMC article. No abstract available.
Abstract
Importance: Concerns have been raised regarding a link between use of glucagon-like peptide-1 (GLP-1) receptor agonists and increased risk of suicidality and self-harm.
Objective: To assess the association between use of GLP-1 receptor agonists and the risk of suicide death in routine clinical practice.
Design, setting, and participants: This active-comparator new-user cohort study used nationwide register data from Sweden and Denmark from 2013 to 2021. Adults 18 to 84 years old who initiated treatment with GLP-1 receptor agonists or the comparator sodium-glucose cotransporter-2 (SGLT2) inhibitors were included. Data were analyzed from March to June 2024.
Exposure: Initiation of treatment with a GLP-1 receptor agonist or SGLT2 inhibitor.
Main outcomes and measures: The primary outcome was suicide death recorded in the cause of death registers. Secondary outcomes were the composite of suicide death and nonfatal self-harm and the composite of incident depression and anxiety-related disorders. Using propensity score weighting, hazard ratios (HRs) with 95% CIs were calculated separately in the 2 countries and pooled in a meta-analysis.
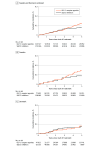
Results: In total, 124 517 adults initiated a GLP-1 receptor agonist and 174 036 initiated an SGLT2 inhibitor; among GLP-1 receptor agonist users, the mean (SD) age was 60 (13) years, and 45% were women. During a mean (SD) follow-up of 2.5 (1.7) years, 77 suicide deaths occurred among users of GLP-1 receptor agonists and 71 suicide deaths occurred among users of SGLT2 inhibitors: weighted incidences were 0.23 vs 0.18 events per 1000 person-years (HR, 1.25; 95% CI, 0.83-1.88), with an absolute difference of 0.05 (95% CI, -0.03 to 0.16) events per 1000 person-years. The HR was 0.83 (95% CI, 0.70-0.97) for suicide death and nonfatal self-harm, and the HR was 1.01 (95% CI, 0.97-1.06) for incident depression and anxiety-related disorders.
Conclusions and relevance: This cohort study, including mostly patients with type 2 diabetes, does not show an association between use of GLP-1 receptor agonists and an increased risk of suicide death, self-harm, or incident depression and anxiety-related disorders. Suicide death among GLP-1 receptor agonist users was rare, and the upper limit of the confidence interval was compatible with an absolute risk increase of no more than 0.16 events per 1000 person-years.
Conflict of interest statement
Figures

Comment on
-
Glucagon-Like Peptide-1 Receptor Agonists and Suicidality-Two Important Pieces of Data but an Incomplete Puzzle.JAMA Intern Med. 2024 Nov 1;184(11):1312-1313. doi: 10.1001/jamainternmed.2024.4320. JAMA Intern Med. 2024. PMID: 39226047 No abstract available.
References
-
- EMA statement on ongoing review of GLP-1 receptor agonists. European Medicines Agency . July 11, 2023. Accessed November 27, 2023. https://www.ema.europa.eu/en/news/ema-statement-ongoing-review-glp-1-rec...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

