Low dose TamOxifen and LifestylE changes for bReast cANcer prevention (TOLERANT study): Study protocol of a randomized phase II biomarker trial in women at increased risk for breast cancer
- PMID: 39226292
- PMCID: PMC11371200
- DOI: 10.1371/journal.pone.0309511
Low dose TamOxifen and LifestylE changes for bReast cANcer prevention (TOLERANT study): Study protocol of a randomized phase II biomarker trial in women at increased risk for breast cancer
Abstract
Background: Breast Cancer (BC) prevention strategies range from lifestyle changes such as increasing physical activity and reducing body weight to preventive drugs like tamoxifen, known to reduce BC incidence in high-risk women. Sex Hormone Binding Globulin (SHBG) is related to BC risk due to its ability to bind circulating estradiol at high affinity and to regulate estradiol action. A study protocol is presented based on the assessment of the effect of different interventions such as tamoxifen at 10 mg every other day (LDT), intermittent caloric restriction (ICR) two days per week, lifestyle intervention (LI, step counter use) and their combination on the modulation of SHBG and several other biomarkers associated to BC.
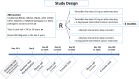
Methods: A randomized phase II biomarker study will be conducted in 4 Italian centers. Unaffected women aged between 18 and 70 years, carriers of a germline pathogenetic variant (BRCA1, BRCA2, PALB2, or other moderate penetrance genes), or with a >5% BC risk at 10 years (according to the Tyrer-Cuzick or the Breast Cancer Surveillance Consortium Risk models) or with a previous diagnosis of intraepithelial neoplasia will be eligible. A total of 200 participants will be randomized to one of the four arms: LDT; LDT + ICR; LI; LI + ICR. Interventions will span six months, with baseline and follow-up clinic visits and interim phone calls.
Discussion: The aim of the study is to verify whether LDT increases circulating SHBG more than LI with or without ICR after 6 months. Secondary objectives include assessing HOMA-index, inflammatory markers, adiponectin/leptin ratio, quality of life (QoL), safety, toxicity, mammographic density, and changes in microbiome composition across groups. The study's innovation lies in its inclusion of diverse BC risk categories and combination of pharmaceutical and behavioral interventions, potentially enhancing intervention efficacy while balancing tamoxifen's side effects on QoL, especially menopausal symptoms.
Trial registration: EuCT number:2023-503994-39-00; Clinical trials.gov NCT06033092.
Copyright: © 2024 Guerrieri-Gonzaga et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous