Energy landscapes of peptide-MHC binding
- PMID: 39226310
- PMCID: PMC11398667
- DOI: 10.1371/journal.pcbi.1012380
Energy landscapes of peptide-MHC binding
Abstract
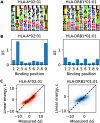
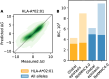
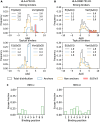
Molecules of the Major Histocompatibility Complex (MHC) present short protein fragments on the cell surface, an important step in T cell immune recognition. MHC-I molecules process peptides from intracellular proteins; MHC-II molecules act in antigen-presenting cells and present peptides derived from extracellular proteins. Here we show that the sequence-dependent energy landscapes of MHC-peptide binding encode class-specific nonlinearities (epistasis). MHC-I has a smooth landscape with global epistasis; the binding energy is a simple deformation of an underlying linear trait. This form of epistasis enhances the discrimination between strong-binding peptides. In contrast, MHC-II has a rugged landscape with idiosyncratic epistasis: binding depends on detailed amino acid combinations at multiple positions of the peptide sequence. The form of epistasis affects the learning of energy landscapes from training data. For MHC-I, a low-complexity problem, we derive a simple matrix model of binding energies that outperforms current models trained by machine learning. For MHC-II, higher complexity prevents learning by simple regression methods. Epistasis also affects the energy and fitness effects of mutations in antigen-derived peptides (epitopes). In MHC-I, large-effect mutations occur predominantly in anchor positions of strong-binding epitopes. In MHC-II, large effects depend on the background epitope sequence but are broadly distributed over the epitope, generating a bigger target for escape mutations due to loss of presentation. Together, our analysis shows how an energy landscape of protein-protein binding constrains the target of escape mutations from T cell immunity, linking the complexity of the molecular interactions to the dynamics of adaptive immune response.
Copyright: © 2024 Collesano et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

