The HIV-1 capsid serves as a nanoscale reaction vessel for reverse transcription
- PMID: 39226318
- PMCID: PMC11398657
- DOI: 10.1371/journal.ppat.1011810
The HIV-1 capsid serves as a nanoscale reaction vessel for reverse transcription
Abstract
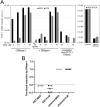
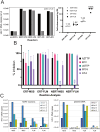
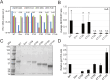
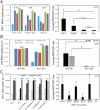
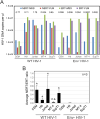
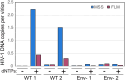
The viral capsid performs critical functions during HIV-1 infection and is a validated target for antiviral therapy. Previous studies have established that the proper structure and stability of the capsid are required for efficient HIV-1 reverse transcription in target cells. Moreover, it has recently been demonstrated that permeabilized virions and purified HIV-1 cores undergo efficient reverse transcription in vitro when the capsid is stabilized by addition of the host cell metabolite inositol hexakisphosphate (IP6). However, the molecular mechanism by which the capsid promotes reverse transcription is undefined. Here we show that wild type HIV-1 virions can undergo efficient reverse transcription in vitro in the absence of a membrane-permeabilizing agent. This activity, originally termed "natural endogenous reverse transcription" (NERT), depends on expression of the viral envelope glycoprotein during virus assembly and its incorporation into virions. Truncation of the gp41 cytoplasmic tail markedly reduced NERT activity, suggesting that gp41 licenses the entry of nucleotides into virions. By contrast to reverse transcription in permeabilized virions, NERT required neither the addition of IP6 nor a mature capsid, indicating that an intact viral membrane can substitute for the function of the viral capsid during reverse transcription in vitro. Collectively, these results demonstrate that the viral capsid functions as a nanoscale container for reverse transcription during HIV-1 infection.
Copyright: © 2024 Jennings et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Update of
-
The HIV-1 capsid serves as a nanoscale reaction vessel for reverse transcription.bioRxiv [Preprint]. 2023 Nov 9:2023.11.08.566350. doi: 10.1101/2023.11.08.566350. bioRxiv. 2023. Update in: PLoS Pathog. 2024 Sep 3;20(9):e1011810. doi: 10.1371/journal.ppat.1011810. PMID: 37986899 Free PMC article. Updated. Preprint.
References
-
- Tang S, Murakami T, Agresta BE, Campbell S, Freed EO, Levin JG. 2001. Human immunodeficiency virus type 1 N-terminal capsid mutants that exhibit aberrant core morphology and are blocked in initiation of reverse transcription in infected cells. J Virol 75:9357–66. doi: 10.1128/JVI.75.19.9357-9366.2001 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

