A Phase II Study of Atezolizumab, Pertuzumab, and High-Dose Trastuzumab for Central Nervous System Metastases in Patients with HER2-Positive Breast Cancer
- PMID: 39226397
- PMCID: PMC11528201
- DOI: 10.1158/1078-0432.CCR-24-1161
A Phase II Study of Atezolizumab, Pertuzumab, and High-Dose Trastuzumab for Central Nervous System Metastases in Patients with HER2-Positive Breast Cancer
Abstract
Purpose: Patients with HER2-positive breast cancer brain metastases have few effective systemic therapy options. In a prior study, pertuzumab with high-dose trastuzumab demonstrated a high clinical benefit rate (CBR) in the central nervous system (CNS) in patients with brain metastases. The current trial evaluated whether the addition of atezolizumab to this regimen would produce further improvements in CNS response.
Patients and methods: This was a single-arm, multicenter, phase II trial of atezolizumab, pertuzumab, and high-dose trastuzumab for patients with HER2-positive breast cancer brain metastases. Participants received atezolizumab 1,200 mg i.v. every 3 weeks, pertuzumab (loading dosage 840 mg i.v., then 420 mg i.v. every 3 weeks), and high-dose trastuzumab (6 mg/kg i.v. weekly for 24 weeks, then 6 mg/kg i.v. every 3 weeks). The primary endpoint was CNS overall response rate per Response Assessment in Neuro-Oncology Brain Metastases criteria. Key secondary endpoints included CBR, overall survival, and safety and tolerability of the combination.
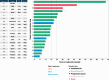
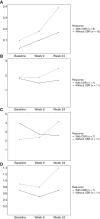
Results: Among 19 enrolled participants, two had a confirmed intracranial partial response for a CNS overall response rate of 10.5% (90% confidence interval, 1.9%-29.6%). The study did not meet the prespecified efficacy threshold and was terminated early. The CBR was 42.1% at 18 weeks and 31.6% at 24 weeks. Seven patients (36.8%) required a dose delay or hold, and the most frequent any-grade adverse events were diarrhea (26.3%) and fatigue (26.3%).
Conclusions: The addition of atezolizumab to pertuzumab plus high-dose trastuzumab does not result in improved CNS responses in patients with HER2-positive breast cancer brain metastases.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
A. Giordano reports personal fees from Pfizer outside the submitted work. P.U. Kumthekar reports personal fees from Seagen, Belay Diagnostics, Plus Therapeutics, Servier, Telix Pharmaceuticals, Biocept, Novocure, Bioclinica, EnClear Therapeutics, Biodexa, BPGbio, and IN8bio and grants and nonfinancial support from Genentech/Roche outside the submitted work. J.P. Leone reports other support from Kazia Therapeutics, Seagen, AstraZeneca, Eli Lilly and Company, and Minerva Biotechnologies outside the submitted work. E.A. Mittendorf reports other support from AstraZeneca, BioNTech, Merck, Moderna, Bristol Myers Squibb, Roche Genentech, and Gilead; personal fees from Merck Sharp & Dohme; and grants from Roche Genentech and Komen for the Cure outside the submitted work. E.L. Mayer reports other support from Eli Lilly and Company, Novartis, and AstraZeneca outside the submitted work. S.M. Tolaney reports grants from Genentech during the conduct of the study, as well as grants and personal fees from Novartis, Merck, AstraZeneca, Genentech/Roche, Eisai, and Bristol Myers Squibb; grants, personal fees, and other support from Pfizer, Eli Lilly and Company, Gilead, and Jazz Pharmaceuticals; personal fees and other support from Sanofi, personal fees from CytomX Therapeutics, Daiichi Sankyo, Zymeworks, Zentalis, Blueprint Medicines, Reveal Genomics, Sumitovant Biopharma, Umoja Biopharma, Artios Pharma, Menarini/Stemline, Aadi Bioscience, Bayer, Incyte Corporation, Natera, Tango Therapeutics, SystImmune, eFFECTOR, Hengrui USA, Cullinan Oncology, Circle Pharma, Arvinas, BioNTech, and Johnson & Johnson; and grants from NanoString Technologies, Seattle Genetics, and OncoPep outside the submitted work. N.U. Lin reports grants from Roche/GNE during the conduct of the study, as well as grants and personal fees from Genentech, Pfizer/Seagen, AstraZeneca, and Olema Pharmaceuticals; grants from Zion Pharmaceuticals and Merck; and personal fees from Stemline/Menarini, Artera Inc., Daiichi Sankyo, Blueprint Medicines, Janssen, and Eisai outside the submitted work. No disclosures were reported by the other authors.
Figures
References
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177–82. - PubMed
-
- Pathmanathan N, Provan PJ, Mahajan H, Hall G, Byth K, Bilous AM, et al. Characteristics of HER2-positive breast cancer diagnosed following the introduction of universal HER2 testing. Breast 2012;21:724–9. - PubMed
-
- Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997–4013. - PubMed
-
- Niwińska A, Tacikowska M, Murawska M. The effect of early detection of occult brain metastases in HER2-positive breast cancer patients on survival and cause of death. Int J Radiat Oncol Biol Phys 2010;77:1134–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

