Ibrutinib plus rituximab and mini-CHOP in older patients with newly diagnosed DLBCL: a phase 2 ALLG study
- PMID: 39226464
- PMCID: PMC11567063
- DOI: 10.1182/bloodadvances.2024014035
Ibrutinib plus rituximab and mini-CHOP in older patients with newly diagnosed DLBCL: a phase 2 ALLG study
Abstract
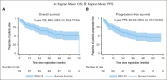
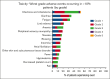
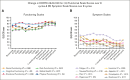
The multicenter, prospective phase 2 Australasian Leukaemia & Lymphoma Group NHL29 trial was conducted to assess the addition of ibrutinib to R-mini-CHOP (dose attenuated R-CHOP; rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) in patients aged ≥75 years with newly diagnosed diffuse large B-cell lymphoma (DLBCL). Treatment consisted of six 21-day cycles of ibrutinib-R-mini-CHOP followed by two 21-day cycles of R-ibrutinib. Coprimary end points were deliverability and 2-year overall survival (OS). The median average relative total dose and average relative dose intensity for the entire regimen were both 97% (interquartile range, 82-100 and 88-100, respectively). With a median follow-up of 35.5 months, the 2-year OS was 68% (95% confidence interval [CI], 55.6-77.4) with a 2-year progression-free survival (PFS) of 60.0% (95% CI, 47.7-70.3). Median OS and PFS were 72 months (95% CI, 35 to not reached) and 40 months (95% CI, 20.4 to not reached), respectively. The overall response rate was 76% (61/79) of patients, with a complete response rate of 71% (56/79). Deaths occurred in 34 of 79 patients (43%), including 17 from progressive disease and 5 treatment related. Overall, 67% patients experienced at least 1 serious adverse event. Most common adverse events were infections and diarrhea (the majority grade 1-2). In both health-related quality of life measures, there was an improvement in functional and symptom scales, median health state classification score, and median visual analogue scale in responders over time. In conclusion, this study showed that the addition of ibrutinib to R-mini-CHOP was both deliverable and efficacious in elderly DLBCL patients.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: E.V. has received research funding paid to her institution for clinical trials from Janssen, BeiGene, AbbVie, Loxo Oncology, and Roche; and reports a role in an advisory board for BeiGene. J.T. has received research funding paid to her institution for clinical trials from Roche, Bristol Myers Squibb, BeiGene, Cellectar, Pharmacyclics and Janssen. T.C. has received research funding from BeiGene. E.A.H. has received research funding paid to her institution from Bristol Myers Squibb, Merck KgaA, AstraZeneca, Roche, and TG Therapeutics; reports a role in an advisory board for Roche, Antengene, Bristol Myers Squibb, Gilead, AstraZeneca, and Regeneron; and serves on a speakers bureau for Regeneron, Janssen, and AstraZeneca. C.Y.C. consults for, serves on advisory boards of, and receives honoraria from Roche, Janssen, Gilead, AstraZeneca, Eli Lilly, BeiGene, Menarini, Dizal, AbbVie, Genmab, and Bristol Myers Squibb; and reports research funding from Bristol Myers Squibb, Roche, AbbVie, Merck Sharp & Dohme, and Eli Lilly. M.K.G. receives study drug from Janssen for a clinical trial. B.E.B. is an independent statistician who consults to a wide variety of pharmaceutical and device companies. The remaining authors declare no competing financial interests.
A complete list of the members of the Australasian Leukaemia & Lymphoma Group involved in this study appears in “Appendix.”
Figures
References
-
- d’Amore F, Brincker H, Christensen BE, et al. Original article: non-Hodgkin’s lymphoma in the elderly: a study of 602 patients aged 70 or older from a Danish population-based registry. Ann Oncol. 1992;3 (5):379–386. - PubMed
-
- Nabhan C, Smith SM, Helenowski I, et al. Analysis of very elderly (≥80 years) non-Hodgkin lymphoma: impact of functional status and co-morbidities on outcome. Br J Haematol. 2012;156(2):196–204. - PubMed
-
- Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60) Lancet Oncol. 2008;9(2):105–116. - PubMed
-
- Thieblemont C, Gomes da Silva M, Casasnovas O, et al. Final analysis of the international double-blind randomized phase III study of lenalidomide maintenance in elderly patients with DLBCL in response after R-CHOP, the Remarc Study from Lysa. Blood. 2020;136(suppl 1):1–2. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials