Analytical Sensitivity Analysis and Clinical Impact Modeling of Rapigen Rapid Diagnostic Tests for Malaria
- PMID: 39226907
- PMCID: PMC11542527
- DOI: 10.4269/ajtmh.24-0003
Analytical Sensitivity Analysis and Clinical Impact Modeling of Rapigen Rapid Diagnostic Tests for Malaria
Abstract
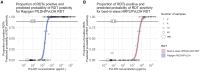
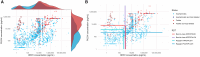
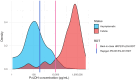
Laboratory benchmarking allows objective analysis of the analytical performance of malaria rapid diagnostic tests (RDTs). We present the analytical detection limits of the Rapigen BIOCREDIT Malaria Ag Pf/Pv (pLDH/pLDH), the Rapigen BIOCREDIT Malaria Ag Pf (pLDH/HRPII), and two best-in-class WHO-prequalified comparator RDTs, generated using standardized panels containing recombinant antigen, in vitro cultured parasites, international standards, and clinical samples. Detection limit antigen concentrations of HRP2, PfLDH, and PvLDH were determined for the Rapigen and comparator RDTs. Detection of antigens in international units (IU)/mL was also evaluated. The Rapigen Ag Pf (pLDH/HRPII) detected 3.9 and 3.9 IU/mL for PfLDH and HRP2, respectively, and the Ag Pf/Pv (pLDH/pLDH) detected 3.9 and 5.0 IU/mL for PfLDH and PvLDH, respectively. The comparator HRP2/PfLDH and HRP2/PvLDH detected 15.6 and 31.3 IU/mL for HRP2 and PfLDH and 15.6 and 50.0 IU/mL for HRP2 and PvLDH, respectively. The RDT clinical sensitivity was predicted through application of analytical detection limits to antigen concentration distributions from clinical symptomatic and asymptomatic cases. Febrile cases would be detected in a majority by both standard and Rapigen RDTs, but incremental increases in sensitivity in the Rapigen RDTs may be important for clinical cases currently missed by microscopy. Rapigen RDTs were predicted to have improved detection of asymptomatic cases and infections with parasites carrying hrp2 deletions through more sensitive PfLDH detection. Through the benchmarking and simulation of clinical sensitivity, a method for rapidly assessing the ability of new RDTs to meet clinical needs using high-sensitivity antigen distribution data is presented.
Figures



References
-
- World Health Organization , 2019. Response Plan to pfhrp2 Gene Deletions. Geneva, Switzerland: WHO.
-
- World Health Organization , 2020. Master Protocol for Surveillance of pfhrp2/3 Deletions and Biobanking to Support Future Research. Geneva, Switzerland: WHO.
-
- Watson OJ, Slater HC, Verity R, Parr JB, Mwandagalirwa MK, Tshefu A, Meshnick SR, Ghani AC, 2017. Modelling the Drivers of the Spread of Plasmodium falciparum hrp2 Gene Deletions in Sub-Saharan Africa. Available at: https://elifesciences.org/articles/25008/figures. Accessed August 24, 2017. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

