Role of the STING→IRF3 Pathway in Ambient GABA Homeostasis and Cognitive Function
- PMID: 39227159
- PMCID: PMC11466066
- DOI: 10.1523/JNEUROSCI.1810-23.2024
Role of the STING→IRF3 Pathway in Ambient GABA Homeostasis and Cognitive Function
Erratum in
-
Erratum: Neupane et al., "Role of the STING→IRF3 Pathway in Ambient GABA Homeostasis and Cognitive Function".J Neurosci. 2025 Jan 15;45(3):e2192242024. doi: 10.1523/JNEUROSCI.2192-24.2024. J Neurosci. 2025. PMID: 39788743 Free PMC article. No abstract available.
Abstract
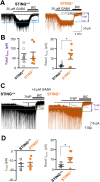
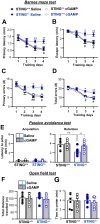
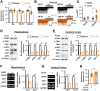
Targeting altered expression and/or activity of GABA (γ-aminobutyric acid) transporters (GATs) provide therapeutic benefit for age-related impairments, including cognitive dysfunction. However, the mechanisms underlying the transcriptional regulation of GATs are unknown. In the present study, we demonstrated that the stimulator of interferon genes (STING) upregulates GAT1 and GAT3 expression in the brain, which resulted in cognitive dysfunction. Genetic and pharmacological intervention of STING suppressed the expression of both GAT1 and GAT3, increased the ambient GABA concentration, and therefore, enhanced tonic GABAA inhibition of principal hippocampal neurons, resulting in spatial learning and working memory deficits in mice in a type I interferon-independent manner. Stimulation of the STING→GAT pathway efficiently restored cognitive dysfunction in STING-deficient mice models. Our study uncovered for the first time that the STING signaling pathway regulates GAT expression in a cell autonomous manner and therefore could be a novel target for GABAergic cognitive deficits.
Keywords: GATs; STING→IRF3; memory; tonic GABA.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
