Towards clinically relevant dose ratios for Cabamiquine and Pyronaridine combination using P. falciparum field isolate data
- PMID: 39227370
- PMCID: PMC11372057
- DOI: 10.1038/s41467-024-51994-3
Towards clinically relevant dose ratios for Cabamiquine and Pyronaridine combination using P. falciparum field isolate data
Abstract
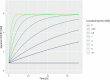
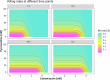
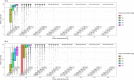
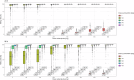
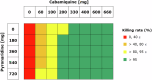
The selection and combination of dose regimens for antimalarials involve complex considerations including pharmacokinetic and pharmacodynamic interactions. In this study, we use immediate ex vivo P. falciparum field isolates to evaluate the effect of cabamiquine and pyronaridine as standalone treatments and in combination therapy. We feed the data into a pharmacometrics model to generate an interaction map and simulate meaningful clinical dose ratios. We demonstrate that the pharmacometrics model of parasite growth and killing provides a detailed description of parasite kinetics against cabamiquine-susceptible and resistant parasites. Pyronaridine monotherapy provides suboptimal killing rates at doses as high as 720 mg. In contrast, the combination of a single dose of 330 mg cabamiquine and 360 mg pyronaridine provides over 90% parasite killing in most of the simulated patients. The described methodology that combines a rapid, 3R-compliant in vitro method and modelling to set meaningful doses for new antimalarials could contribute to clinical drug development.
© 2024. The Author(s).
Conflict of interest statement
C.D.G., C.O., P.C., and T.S. are employed by Ares Trading S.A., Switzerland, an affiliate of the healthcare business of Merck KGaA, Darmstadt, Germany. S.W. consultancy was funded by the healthcare business of Merck KGaA, Darmstadt, Germany. The remaining authors declare no competing interests.
Figures


References
-
- World Health Organization. WHO Malaria Report 2019. Malaria Report 2019 (WHO, 2019).
-
- World Health Organization. WHO Malaria Report 2022 (WHO, 2022).
-
- van der Pluijm, R. W. et al. Determinants of dihydroartemisinin-piperaquine treatment failure in Plasmodium falciparum malaria in Cambodia, Thailand, and Vietnam: a prospective clinical, pharmacological, and genetic study. Lancet Infect. Dis.19, 952–961 (2019). 10.1016/S1473-3099(19)30391-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

